Key Points
- A group of researchers analyzed MRI, histology, and harmonic dose data to determine that the use of microbubbles and focused ultrasound was safe for opening the blood-brain barrier (BBB) in patients with infiltrating gliomas.
- Data from the Phase 0 trial showed a significant, 2.2-fold increase of fluorescein accumulation in tumor tissues that were treated with microbubbles and focused ultrasound.
Localized Blood–Brain Barrier Opening in Infiltrating Gliomas with MRI-Guided Acoustic Emissions–Controlled Focused Ultrasound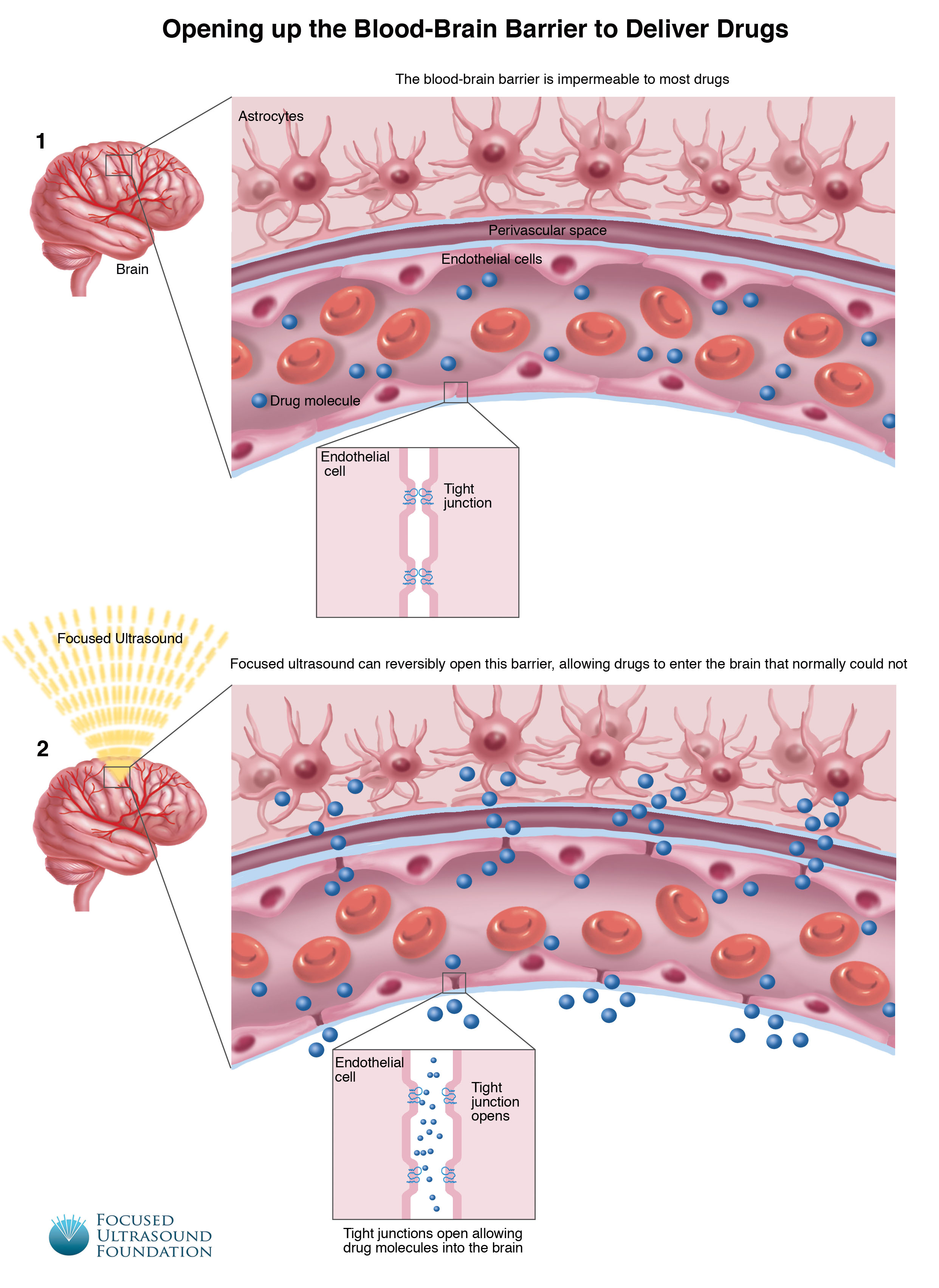
A group of collaborative researchers from the University of Maryland and the Georgia Institute of Technology analyzed MRI, histology, and harmonic dose data to determine whether the use of microbubbles and focused ultrasound was safe for opening the blood-brain barrier (BBB) in patients with infiltrating gliomas. The Phase 0 clinical trial (NCT03322813) used comparative and quantitative analyses and fluorescence-guided surgery metrics. The data showed a significant, 2.2-fold increase of fluorescein accumulation in tumor tissues that were treated with microbubbles and focused ultrasound as compared with untreated tumors (P < 0.01). Vascular density was a factor. The group concluded that focused ultrasound could provide “safe, localized, controlled, and tightly monitored BBB opening through the intact skull.”
See the Proceedings of the National Academy of Sciences (PNAS) >
A multicenter pivotal clinical trial with 120 participants is now being planned.
