Key Points
- Profound Medical’s Sonalleve® system has received the CE mark for the treatment of desmoid tumors.
- Desmoid tumors are benign but aggressive soft tissue tumors that typically occur in young adults.
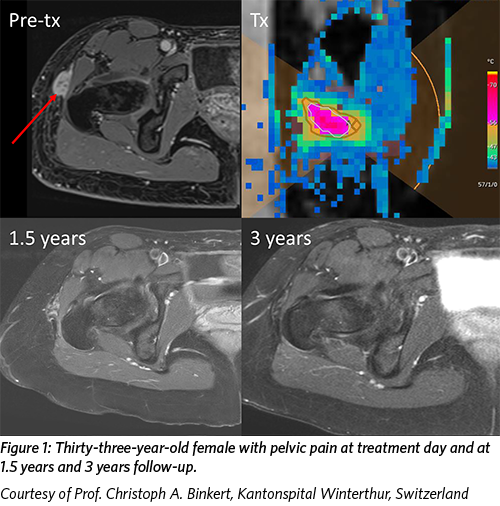 Profound Medical recently announced that its Sonalleve® MR-guided High Intensity Focused Ultrasound (MR-HIFU) system has received the CE mark for the treatment of desmoid tumors.
Profound Medical recently announced that its Sonalleve® MR-guided High Intensity Focused Ultrasound (MR-HIFU) system has received the CE mark for the treatment of desmoid tumors.
Desmoid tumors are benign but aggressive soft tissue tumors that typically occur in young adults. Patients with advanced desmoid tumors usually experience pain, swelling, and/or loss of function in the affected area. Traditional treatment options include radiofrequency ablation and surgery, although both carry unique risks for patients.
Sonalleve® combines real-time MR imaging and thermometry with thermal ultrasound to enable precise, noninvasive ablation of diseased tissue. The treatment offers patients with desmoid tumors a treatment that can be performed safely with clinical improvement without the risks of surgery, invasive procedures, ionizing radiation exposure, or anesthesia.
“Based on our experience, MR-HIFU offers a valuable treatment option for the often difficult to treat desmoid tumors,” said Dr. Manon Braat, a radiologist at UMC Utrecht in the Netherlands. “I believe that the potential benefit of MR-HIFU treatment does outweigh the risks for patients, especially since MR-HIFU treatment can be repeated in case of recurrent disease with limited side-effects (contrary to the conventional treatment methods).”
“We’re really pleased that we can add another critical application to our MR-HIFU Platform Sonalleve, which complements the recent CE mark and FDA HDE approval for osteoid osteomas,” said Dr. Arun Menawat, CEO of Profound Medical. “We’re now looking forward to working with our global clinical partners to drive clinical adoption to establish both applications as a favorable alternative to the current standard of care for pediatric patients and young adults.”
The Sonalleve® system also has CE mark to treat pain from osteoid osteomas, metastatic bone cancer, uterine fibroids, and adenomyosis.
In the US, the device was approved under the Food and Drug Administration’s Humanitarian Device Exemption to treat osteoid osteomas – benign, painful bone tumors – in November 2020.
