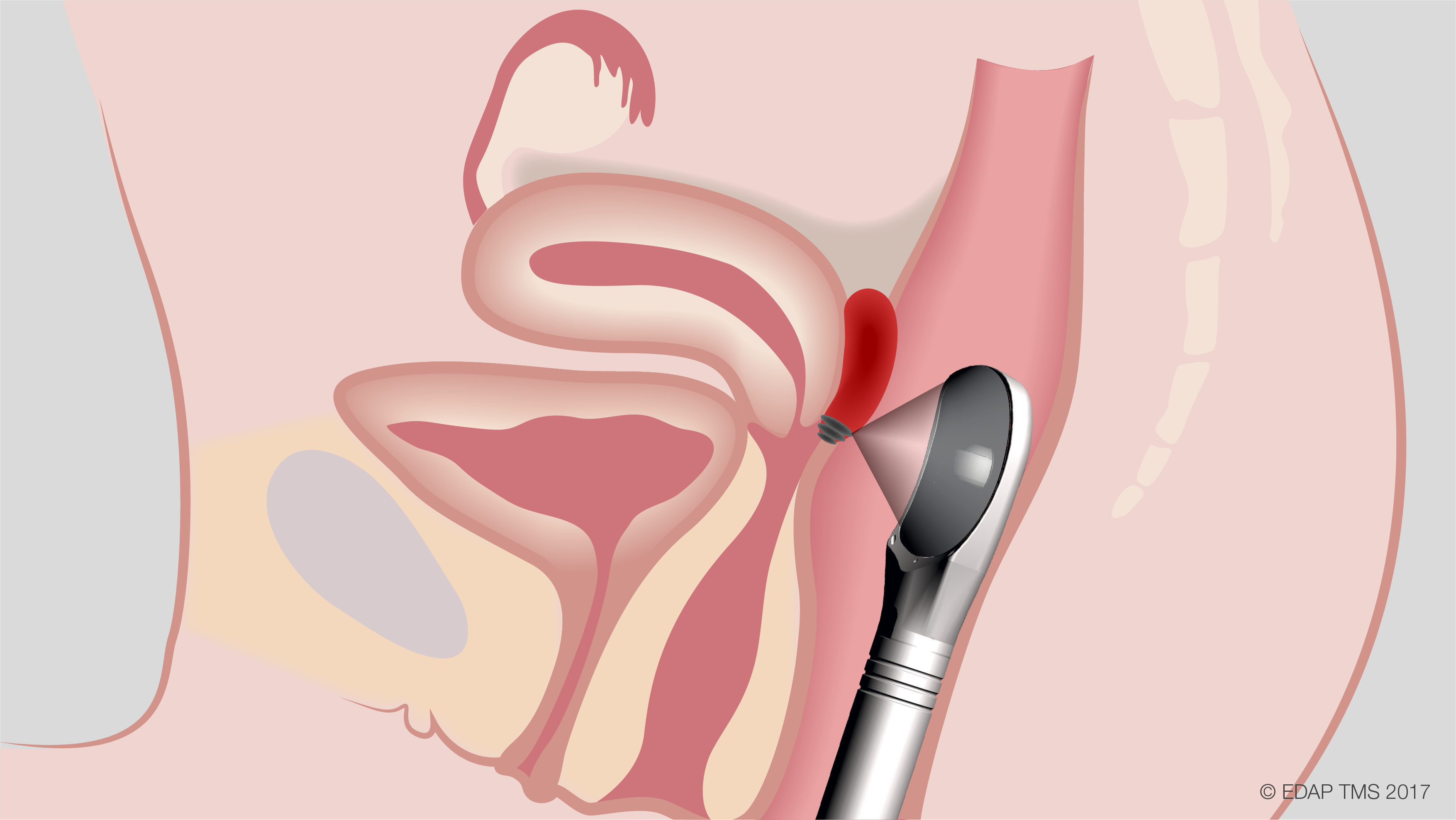
 EDAP TMS recently announced that the first two patients with deep invasive endometriosis of the rectosigmoid area have been treated in a Phase 2 clinical trial assessing the safety and efficacy of using their Focal One® high intensity focused ultrasound (HIFU) device to treat the condition. The trial will take place at five major hospitals in France, with a goal to treat and follow at least 38 female patients for six months.
EDAP TMS recently announced that the first two patients with deep invasive endometriosis of the rectosigmoid area have been treated in a Phase 2 clinical trial assessing the safety and efficacy of using their Focal One® high intensity focused ultrasound (HIFU) device to treat the condition. The trial will take place at five major hospitals in France, with a goal to treat and follow at least 38 female patients for six months.
Endometriosis affects approximately 176 million women worldwide, and digestive endometriosis affects 20% of these women. When medical treatments are ineffective, surgical resection remains the standard of care. Researchers are hopeful that Focal One® HIFU – a minimally invasive, transrectal procedure that has US Food and Drug Administration approval for the ablation of prostate tissue – may prove to be a better option for these patients.
The current trial builds upon data from an earlier Phase I clinical study, during which 20 patients with the disease were observed to benefit from Focal One® treatment. The full results of the study were published in Ultrasound in Obstetrics & Gynecology in December 2019.
Both the Phase I and the current trial are led by Professor Gil Dubernard, Head of the Gynecologic Department at Croix-Rousse University Hospital in Lyon, France.
“Endometriosis, particularly the deep invasive form, can be a painful and debilitating disease that is very much in need of new treatment options,” stated Pr. Dubernard. “Based on our earlier findings in a Phase I trial, I believe HIFU holds great promise as an effective and minimally invasive treatment. This trial will raise awareness of the potential of HIFU across many high-need indications in France and elsewhere.”
“The initiation of this endometriosis trial is consistent with our recent strategic focus to further expand our HIFU pipeline into new indications,” noted Marc Oczachowski, EDAP’s Chairman and CEO. “As a leading innovator in the field of HIFU ablation, we see it as our mission to make this technology to the broadest possible patient population.”
