|
Dear Friends,
 |
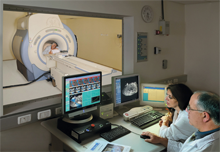 |
| A patient with painful bone metastases undergoing palliative therapy with ExAblate® MRI-guided focused ultrasound therapy. |
"It's all about patients," is a guiding principle of the Focused Ultrasound Foundation. Among the patients in urgent need of new treatment options are those living in excruciating pain caused by metastatic bone cancer. This is a late-stage malignancy that results from the spread of other forms of cancer, such as breast, prostate, lung, bladder and thyroid. While radiation therapy is a standard of care for these patients, one out of every three of them experiences no benefit or cannot undergo additional radiation exposure.
This week, the FDA approved InSightec's ExAblate system to provide palliative treatments for metastatic bone cancer. The approval offers an important new pain-management option for patients who are not responsive to or not eligible for radiation, and it is a development that we applaud.
 |
 |
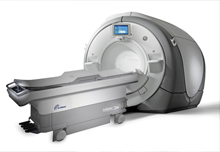
| InSightec's ExAblate, a new, non-invasive, non-ionizing, FDA-approved therapy to treat pain from bone metastases in patients who cannot undergo radiation. |
In many respects, the ExAblate system has led the way for the field of focused ultrasound, especially in the U.S., which many still view as the holy grail of healthcare markets. The system, which received FDA approval in 2004 to treat symptomatic uterine fibroids, remains the only focused ultrasound device cleared for clinical use in the U.S. The ExAblate also has received European CE marking for uterine fibroids, bone metastases, and adenomyosis and is providing patient treatments in many countries around the world.
We congratulate the team at InSightec and the many researchers at clinical sites in the U.S. and elsewhere who made this important regulatory approval possible. On behalf of the many patients who will benefit from this new treatment option, we say, "thank you."

Read InSightec's press release >
Access videos and additional background information >
|