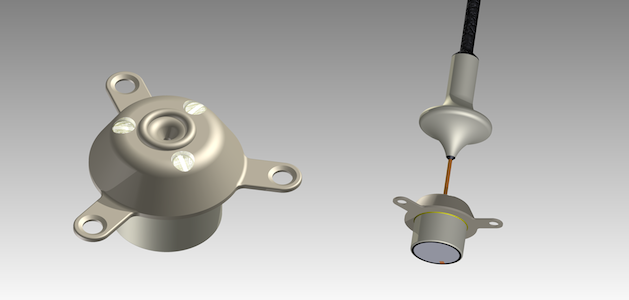
French medical device start-up CarThera recently published the complete results from its Phase I/IIa clinical trial using the SonoCloud-1 ultrasound implant to open the blood-brain barrier (BBB) prior to carboplatin chemotherapy in patients with recurrent glioblastoma (GBM).
Study results, which were published in the journal Clinical Cancer Research, included a good safety profile and “encouraging efficacy.” The trial included 19 patients who received up to ten monthly sonications at escalating pressure levels. Patients treated at pressure levels that were optimal for disrupting the BBB had improved survival.
 The 11 GBM patients treated with optimal pressure levels showed a median progression-free survival (PFS) of 4.11 months and a median overall survival (OS) of 12.94 months. The remaining eight patients showed a PFS of 2.73 months and an OS of 8.64 months.
The 11 GBM patients treated with optimal pressure levels showed a median progression-free survival (PFS) of 4.11 months and a median overall survival (OS) of 12.94 months. The remaining eight patients showed a PFS of 2.73 months and an OS of 8.64 months.
“This trial clearly shows the potential of the SonoCloud technology to enhance the brain penetration of both existing and new drugs for GBM and other brain diseases,” said CarThera founder Professor Alexandre Carpentier. “This is the first trial in the world presenting the feasibility and safety results of iterative BBB opening with significant indications for better tumor control and preserved neurological conditions.”
CarThera is now recruiting patients with recurrent GBM for a clinical trial (NCT03744026) that uses the SonoCloud-9 implantable ultrasound  device. This next-generation system is designed to cover a larger volume of the brain, including the GBM tumor and its surrounding infiltrative regions.
device. This next-generation system is designed to cover a larger volume of the brain, including the GBM tumor and its surrounding infiltrative regions.
See the CarThera Press Release >
See the publication in Clinical Cancer Research >
See related story: CarThera Company Profile >
