The Foundation thanks Joan Vidal-Jové, MD, PhD, of the Interventional and Surgical Oncology department at the Comprehensive Tumor Center Barcelona in Barcelona, Spain, for providing this case report.
The Patient
In November 2013, a 67-year-old male with stage 4 colon cancer underwent surgical resection of a part of his colon containing cancer. During the operation, he was noted to have hepatic metastases. After recovery from his colon surgery, the patient was started on chemotherapy. In 2014 and 2015, he underwent two separate open surgeries to remove liver masses, and chemotherapy was continued. From 2015 to 2018, his cancer was in remission, and he was maintained on adjuvant chemotherapy. In May 2018, during routine follow-up, the patient showed marked progression of his liver tumors in addition to new lung masses. Due to these findings, he was started on a different chemotherapy regimen plus an immunotherapy drug. In July 2018, three of the patient’s liver masses were treated with thermal ablation by ultrasound-guided, high-intensity focused ultrasound (HIFU). He had a partial response to this treatment, but, unfortunately, his disease continued to progress, and he was maintained on the chemotherapy plus immunotherapy regimen.
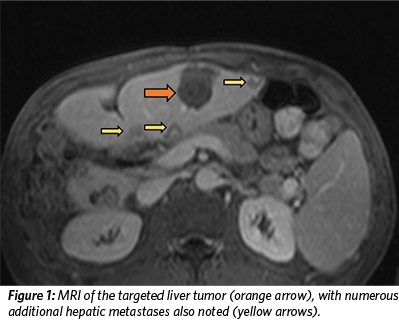 In April 2019, an MRI of the patient’s abdomen revealed countless liver masses that had increased in number and size since his last evaluation (Figure 1). The patient received an evaluation by the Tumor Board at Comprehensive Tumor Center Barcelona, and his inclusion in a clinical trial – the THERESA study – was approved. The THERESA study is a first-in-human clinical trial sponsored by HistoSonics, Inc. to establish the safety and efficacy of their histotripsy device to treat liver tumors. The patient was not considered a candidate for other surgical or locoregional therapies.
In April 2019, an MRI of the patient’s abdomen revealed countless liver masses that had increased in number and size since his last evaluation (Figure 1). The patient received an evaluation by the Tumor Board at Comprehensive Tumor Center Barcelona, and his inclusion in a clinical trial – the THERESA study – was approved. The THERESA study is a first-in-human clinical trial sponsored by HistoSonics, Inc. to establish the safety and efficacy of their histotripsy device to treat liver tumors. The patient was not considered a candidate for other surgical or locoregional therapies.
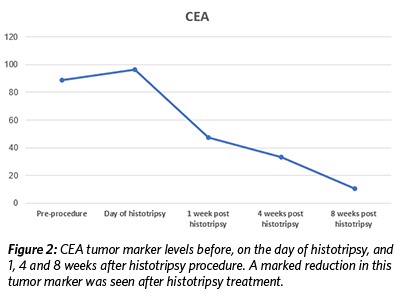
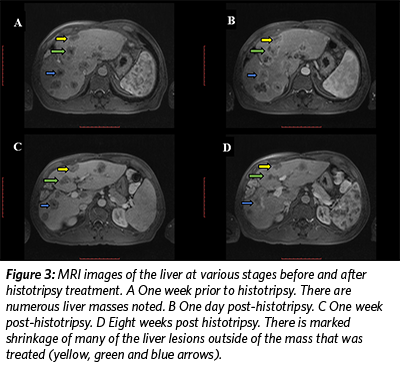
On May 13, 2019, the patient underwent histotripsy-mediated ablation of a 1.2 cm x 1 cm lesion in his liver. No adverse events occurred during or after the procedure, including no pain. In the weeks following the procedure, lab tests revealed a significant decrease in the value of the tumor marker CEA (Figure 2), and the patient continued to feel well with no pain. Follow up MRI scans at one, four, and eight weeks showed a decrease in size of the targeted, treated lesion as well as a decrease in size of numerous other, non-treated lesions throughout the liver (Figure 3). Immune assessment (CD3, CD4, CD8, IL6, Complement) was equivocal.
 Following the advice of the patient’s oncologist, a new chemotherapy regime was initiated five weeks after the ablation procedure. After this, both tumor marker levels and the size of the liver lesions continued to decrease.
Following the advice of the patient’s oncologist, a new chemotherapy regime was initiated five weeks after the ablation procedure. After this, both tumor marker levels and the size of the liver lesions continued to decrease.
In December 2019, there was progression of the liver metastases and appearance of a new tumor in the colon. New treatment options and clinical trials are still being explored. At present, one year after his treatment with histotripsy and evidence of an abscopal effect, the patient has slow progression of his disease.
Focused Ultrasound for Abdominal Tumors
Surgical resection is the established first-line treatment for primary and metastatic liver cancer. However, surgical removal with curative intent is only feasible for a minority of patients with liver metastases (10-25 percent) since only a small proportion of patients have tumors that are entirely resectable at presentation.1 Despite the survival advantage of hepatic resection on colorectal cancer liver metastases, relapse is common following curative resection.2 In addition, surgery is an invasive procedure associated with high rates of morbidity and mortality.3
 Ablation techniques are promising alternatives for those patients who are not eligible for surgical resection or who have failed other therapies. Current ablation methods include non-thermal ablation methods (e.g., percutaneous ethanol injection [PEI] and irreversible electroporation [IRE]) and thermal modalities (e.g., radiofrequency ablation [RFA, microwave ablation [MWA], and HIFU.)4
Ablation techniques are promising alternatives for those patients who are not eligible for surgical resection or who have failed other therapies. Current ablation methods include non-thermal ablation methods (e.g., percutaneous ethanol injection [PEI] and irreversible electroporation [IRE]) and thermal modalities (e.g., radiofrequency ablation [RFA, microwave ablation [MWA], and HIFU.)4
Despite the efficacy of some of these local thermal ablation modalities, significant limitations exist due to their mode of action (thermal tissue destruction). Thermal ablation is inconsistent in tissue with non-uniform heat dissipation patterns, which is common in liver tumors.5 It often results in incomplete tumor necrosis in tissue that is located near major vessels.6, 7 Consequently, the shape and the size of the ablation zone may be unpredictable, and the efficacy of thermal ablation may be restricted.8 In addition, thermal ablation methods are often unsuitable for treating tumors larger than three centimeters due to excessive treatment time and practical ultrasound probe sizes.9-11 Most complications associated with RFA and MWA are consequences related to thermal injury.12 Another limitation of these methods is the lack of imaging feedback during treatment. Thus, CT or MRI evaluates the effect of ablation treatment after the application of thermal treatment while no real-time imaging provides monitoring during treatment.13
HIFU is a noninvasive, image-guided, thermal ablation method. Unlike percutaneous thermal modalities, HIFU is completely extracorporeal and lacks the risks of bleeding and tumor seeding with the direct puncture of tumors. HIFU can improve upon other thermal ablation modalities due to its noninvasiveness, real-time feedback, and the ability to scan the focal zone over a large volume.13 As with the other thermal-based methods, HIFU is limited by the heat-sink effect, resulting in reduced efficacy in ablating tissue near major vessels and by extended treatment time for larger liver volumes.13 Another major challenge in the noninvasive treatment of liver tumors using HIFU is rib obstruction, which may result in secondary hot spots near the treatment main focal zone, inducing loss of therapeutic precision and collateral damage.14 Moreover, because of the high ultrasound absorption coefficient at the bone-tissue interface, overheating of ribs and surrounding tissue often results in unwanted tissue damage. Skin burns and subcostal edema have been reported with HIFU ablation cases.15, 16
Therefore, developing new strategies in which a liver tumor can be ablated noninvasively and avoiding thermal-related collateral damage and inefficacy would be a major clinical advancement. To address this unmet clinical need, cavitation-based, ultrasound-guided treatment (histotripsy) is a promising option to destroy liver tumors and overcome the limitations of currently available ablation modalities.
Histotripsy is a treatment technology that mechanically destroys targeted tissue through the precise targeting of acoustic cavitation.17-19 The ablation system is an image-guided device designed to deliver noninvasive, non-thermal histotripsy for local treatment that has the potential to overcome many limitations of other focal liver tumor treatment options.
The Histotripsy Group in the Biomedical Engineering Department at University of Michigan invented and pioneered the development of focused ultrasound histotripsy more than 12 years ago. Starting with their earliest work with the use of microbubbles to cause tissue damage, this group developed histotripsy into a highly controlled and predictable tool to remove unwanted tissue with microscopic precision. In 2010, HistoSonics, Inc. entered into a worldwide exclusive license with the University of Michigan for exclusive rights to the entire portfolio of histotripsy patents and patent applications.
Favorable characteristics of histotripsy treatment method include:
- No insertion of probes or needle electrodes required
- Ultrasound imaging feedback for pre-operative planning and real-time visualization of target tumor and image-guided tissue destruction
- Prevents damage to adjacent structures
- Overcomes rib obstruction
- Precise targeting
- Minimal scarring
An additional potential benefit of histotripsy may be as immunogenic ablation20 if it can be used to stimulate tumor-specific immune responses capable of magnifying the impact of checkpoint inhibition immunotherapy. The characteristics of this cavitation-based ablation likely allow cytokines and metabolites – not destroyed in the tumor micro-environment – to become highly immunogenic and contribute to the abscopal effect, where shrinkage of untargeted tumors occurs secondary to an immune response.
The abscopal – or “off target” – effect was first described in patients who were receiving radiation therapy that were noted to have regression of tumors that were in a non-irradiated zone. It describes the ability of localized radiation to initiate an antitumor response that kills cancer cells distant to the primary target. Similar to radiation, focused ultrasound has been shown to produce an abscopal effect in both preclinical and human cancers. When combined with immunotherapy, the abscopal effect could produce a durable treatment response to control or eradicate metastatic cancer.
Conclusion and Future Goals
This case report shows clear evidence of an immunologic relationship between histotripsy ablation and the abscopal effect. A patient with progressive and extensive metastatic disease with a short overall survival prognosis had noticeable shrinkage of non-targeted metastases and is still alive and considering new clinical trial options one year after the histotripsy procedure.
In addition, this report highlights the differences between two focused ultrasound modalities. Thermal US guided HIFU was performed previously and obtained a substantial volume ablation but no immune effects. Less volume ablation with histotripsy generated a noticeable abscopal effect, and this data will influence future research assumptions.
Histotripsy is a disruptive technology. The non-thermal and noninvasive characteristics of histotripsy offer patients the potential for a tumor treatment with fewer clinical complications and adverse events than currently available ablation methods and surgical procedures. The safety of histotripsy has been demonstrated through rigorous testing including benchtop and both acute and chronic disease preclinical studies. Future clinical trials with the objectives to evaluate technical performance, including acute technical success, while collecting safety-related data are forthcoming. In addition, further clinical trials should continue to explore histotripsy-mediated immune effects in detail.
The THERESA Study used an investigative histotripsy device that is not yet commercially available. The THERESA Study is currently ongoing; therefore, data is not considered final.
References
- Wicherts DA, de Haas RJ, Adam R. Bringing unresectable liver disease to resection with curative intent. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007;33 Suppl 2:S42-51. Epub 2007/11/06.
- Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Annals of surgery. 2007;246(2):183-91. Epub 2007/08/02.
- Livraghi T, Makisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scandinavian journal of surgery: SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2011;100(1):22-9. Epub 2011/04/13.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208-36. Epub 2005/10/27.
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441-51. Epub 2003/02/04.
- Aschoff AJ, Merkle EM, Wong V, Zhang Q, Mendez MM, Duerk JL, et al. How does alteration of hepatic blood flow affect liver perfusion and radiofrequency-induced thermal lesion size in rabbit liver? Journal of magnetic resonance imaging: JMRI. 2001;13(1):57-63. Epub 2001/02/13.
- Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113-24. Epub 2010/07/17.
- Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Annals of surgery. 2005;242(2):158-71. Epub 2005/07/26.
- Curley SA. Radiofrequency ablation of malignant liver tumors. The oncologist. 2001;6(1):14-23. Epub 2001/02/13.
- Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. Journal of vascular and interventional radiology: JVIR. 2003;14(10):1267-74. Epub 2003/10/11.
- Marrero JA, Pelletier S. Hepatocellular carcinoma. Clinics in liver disease. 2006;10(2):339-51, ix. Epub 2006/09/15.
- Lahat E, Eshkenazy R, Zendel A, Zakai BB, Maor M, Dreznik Y, et al. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary surgery and nutrition. 2014;3(5):317-23. Epub 2014/11/14.
- Vlaisavljevich E, Kim Y, Allen S, Owens G, Pelletier S, Cain C, et al. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound in medicine & biology. 2013;39(8):1398-409. Epub 2013/05/21.
- Bobkova S, Gavrilov L, Khokhlova V, Shaw A, Hand J. Focusing of high-intensity ultrasound through the rib cage using a therapeutic random phased array. Ultrasound in medicine & biology. 2010;36(6):888-906. Epub 2010/06/01.
- Jung SE, Cho SH, Jang JH, Han JY. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdominal imaging. 2011;36(2):185-95. Epub 2010/06/01.
- Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrasonics sonochemistry. 2004;11(3-4):149-54. Epub 2004/04/15.
- Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound in medicine & biology. 2006;32(1):115-29. Epub 2005/12/21.
- Roberts WW, Hall TL, Ives K, Wolf JS, Jr., Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. The Journal of urology. 2006;175(2):734-8. Epub 2006/01/13.
- Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, et al. Controlled ultrasound tissue erosion. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2004;51(6):726-36. Epub 2004/07/13.
- Shibin Qu, Tejaswi Worlikar, Amy E Felsted, Anutosh Ganguly, Megan V Beems, Ryan Hubbard, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 2020; 8:e000200. doi:10.1136/ jitc-2019-000200
