The traditional path to bring new medical therapies from the laboratory bench to the patients’ bedside is relatively straight: Test first in cell culture, then in small laboratory animals, next in larger laboratory animals, and finally in humans. However, this process has resulted in a spectacularly high failure rate. Recent studies indicate that less that 10 percent of drugs entering clinical trials receive FDA approval – and that success rate is even lower for chemotherapeutics. Why do so many drugs, particularly chemotherapies, fail in clinical trials?
One significant weakness in the development pipeline is the preclinical data used to determine whether a specific drug warrants a clinical trial. This crucial evidence usually comes from small animal studies – in other words, the mouse. Laboratory mice live extremely well-controlled lives: Their diets and environments are sterilized and standardized. Their light exposure and temperature are carefully regulated. Mice in a study are the same age and are virtually indistinguishable genetically. All of this is intentional – it reduces the number of variables and confounding factors, simplifying data analysis. But does this decades-old system do more harm than good?
In short, it might. Laboratory temperatures became a ‘hot’ topic recently after several groups demonstrated that tumor growth and responses to various chemotherapeutics, particularly immunotherapies, changed drastically when room temperatures were increased by seven degrees Centigrade. Other studies indicated that mice with natural gut microbes – produced by a varied diet that laboratory mice do not receive – better replicate human responses to therapies than their laboratory cousins. How can we improve on this system?
The answer may be sitting right beside you! Our companion animals are exposed to the same environments we are – car exhaust, lawn care chemicals, household cleaners, pizza on weekends – and develop many of the same diseases. They are genetically diverse, with natural gut microbes and immune systems from a life lived outside the laboratory. Not surprisingly, our companion animals often respond to medical therapies in much the same way we do. Clinical trials in these animals benefit both humans and animals. The creation of unique ‘integrated comparative clinical trials’ promises to accelerate progress in veterinary and human medicine.
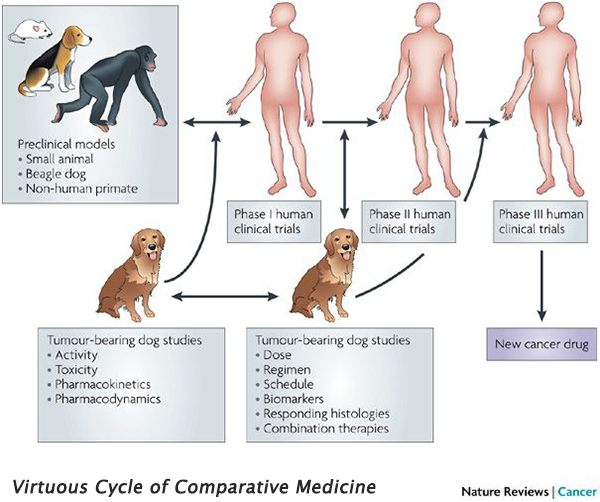 Integrated comparative clinical trials have two distinct differences when compared to traditional clinical trials: They consist of human and companion animal (usually dog) studies running in parallel, and they are iterative. These trial designs are particularly beneficial in diseases which are more common in dogs than in humans (e.g., osteosarcoma), and diseases with poor mouse models (e.g., glioblastoma). Drugs can be rapidly screened in dogs, and the most promising candidates are quickly translated to human clinical trials. Information gathered from the human trial is then applied to further studies in dogs, creating an iterative feedback loop that benefits both species – the ‘virtuous cycle’ of comparative medicine.
Integrated comparative clinical trials have two distinct differences when compared to traditional clinical trials: They consist of human and companion animal (usually dog) studies running in parallel, and they are iterative. These trial designs are particularly beneficial in diseases which are more common in dogs than in humans (e.g., osteosarcoma), and diseases with poor mouse models (e.g., glioblastoma). Drugs can be rapidly screened in dogs, and the most promising candidates are quickly translated to human clinical trials. Information gathered from the human trial is then applied to further studies in dogs, creating an iterative feedback loop that benefits both species – the ‘virtuous cycle’ of comparative medicine.
The diversity and complexity of tumors that occur naturally in our companion animals cannot be replicated in the artificial environment of the laboratory, and access to canine patients has enabled the study of complex biological pathways. For example, our immune systems are so individualized that predicting responses to immunotherapies can be difficult. A combination of two immunotherapies, Interleukin-2 and -12, was evaluated in dogs, and the results informed human trials. Precision medicine – the practice of tailoring treatments to a specific patient using genetic information – has also received a boost from comparative trials, since it relies on genetic variations not seen in lab-grown tumors. These integrated comparative clinical trials have been supported by various entities, including the One Health Initiative, the Comparative Oncology Trials Consortium, and the Canine Comparative Oncology & Genomics Consortium. The work from these organizations has been transformative for both veterinary and human medicine.
The Focused Ultrasound Foundation is interested in integrated comparative clinical trials, and has funded several comparative trials to treat cancers like sarcoma, melanoma, and hepatocellular carcinoma that affect both dogs and humans.
Additional Resources:
Veterinary Program
Focused Ultrasound Foundation Launches Veterinary Program
Foundation Funds Veterinary Clinical Trials
Kelsie Timbie, PhD, is the Scientific Programs Manager and the Veterinary Program Director at the Focused Ultrasound Foundation.
