Key Points
- A research team from the University of Virginia and Stanford University has published innovative preclinical research for treating neurological disorders in the Journal of Neurosurgery.
- Their treatment method called “precise intracerebral noninvasive guided (PING) surgery” uses MR-guided, low-intensity focused ultrasound plus microbubbles to open the blood-brain barrier and deliver a neurotoxin to a targeted area of the brain.
Groundbreaking preclinical research for treating epilepsy and other brain disorders was recently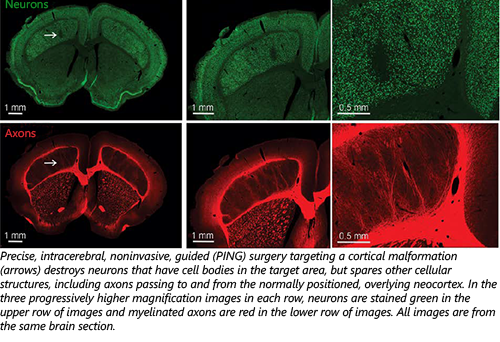 published in the Journal of Neurosurgery (JNS). In “Noninvasive Disconnection of Targeted Neuronal Circuitry Sparing Axons of Passage and Nonneuronal Cells,” a group of clinicians and scientists based at the University of Virginia and Stanford University tested a novel treatment method called “precise intracerebral noninvasive guided (PING) surgery.” PING uses MR-guided, low-intensity focused ultrasound plus microbubbles to open the blood-brain barrier (BBB) and precisely deliver a neurotoxin (quinolinic acid) to the brain. The objective of the PING procedure is to eliminate neurons that are causing problems in the brain.
published in the Journal of Neurosurgery (JNS). In “Noninvasive Disconnection of Targeted Neuronal Circuitry Sparing Axons of Passage and Nonneuronal Cells,” a group of clinicians and scientists based at the University of Virginia and Stanford University tested a novel treatment method called “precise intracerebral noninvasive guided (PING) surgery.” PING uses MR-guided, low-intensity focused ultrasound plus microbubbles to open the blood-brain barrier (BBB) and precisely deliver a neurotoxin (quinolinic acid) to the brain. The objective of the PING procedure is to eliminate neurons that are causing problems in the brain.
“The toxin is well tolerated when administered systemically,” said Kevin S. Lee, PhD, a professor of neuroscience and neurosurgery at the University of Virginia. “It destroys the faulty neuron cell bodies that are in the region of the BBB opening but spares healthy axons passing through the targeted area. For example, in patients with drug-resistant temporal lobe epilepsy, PING could destroy the aberrant temporal lobe neurons but spare adjacent visual system fibers. In this way, seizures would be suppressed but the common surgical complication of permanent visual system deficits would be avoided.”
In their experiments on rats, the research team led by Dr. Lee and Max Wintermark, MD, a professor of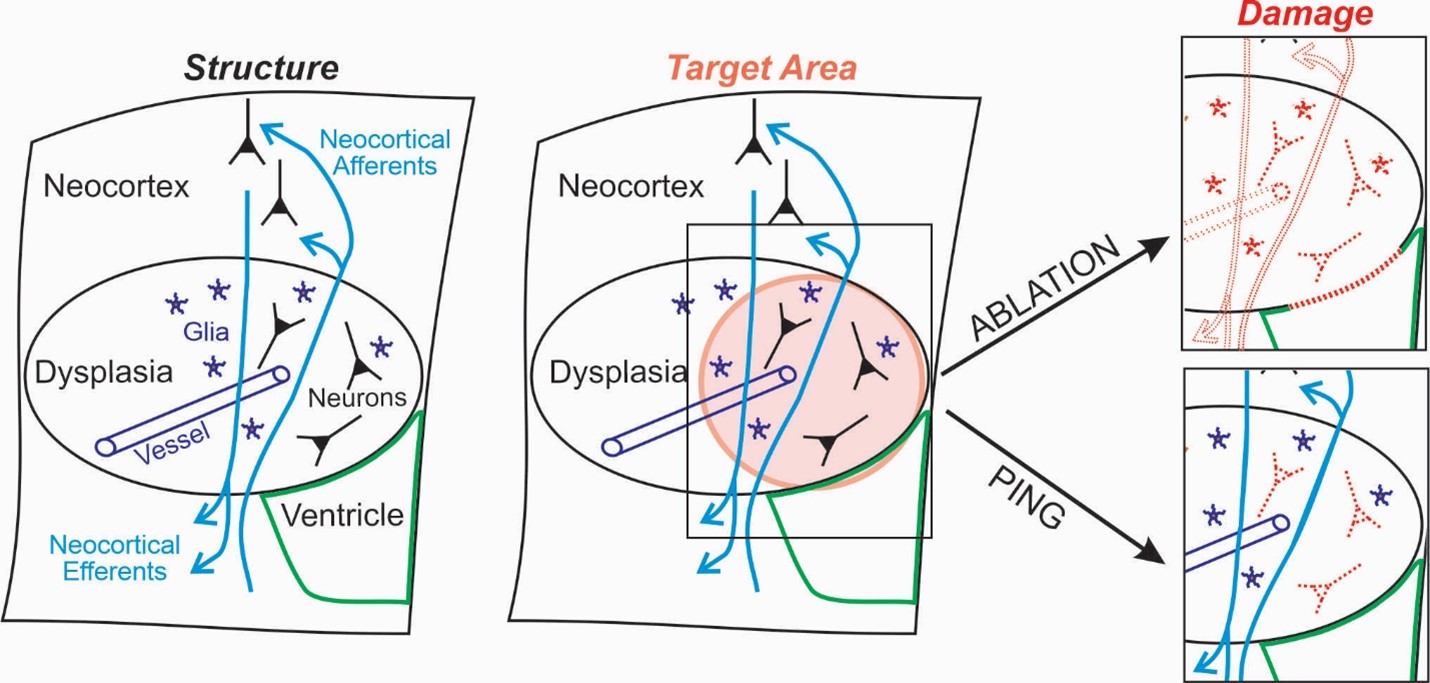 radiology at Stanford University, used the PING procedure to open the BBB in regions of the brain that are typically targeted in epilepsy surgery. Delivering quinolinic acid with PING created precise neuronal loss and circuit disconnection while preserving other important brain structures in the target zone, such as axons of passage, glial cells, blood vessels, and the ventricular wall.
radiology at Stanford University, used the PING procedure to open the BBB in regions of the brain that are typically targeted in epilepsy surgery. Delivering quinolinic acid with PING created precise neuronal loss and circuit disconnection while preserving other important brain structures in the target zone, such as axons of passage, glial cells, blood vessels, and the ventricular wall.
Importantly, if it is possible to translate this research to humans, PING could augment or replace existing ablative or resective surgical techniques that produce comprehensive brain tissue damage. And, it would expand the treatment envelope by allowing access to a broader range of brain areas, including regions that are off limits to current surgical approaches. An additional advantage is that PING can be used to treat irregularly shaped surgical targets, which are commonly encountered in epilepsy surgery. PING is therefore a promising treatment for some of the one-third of epilepsy patients who do not respond to anti-seizure drugs because it holds the potential to reduce or eliminate seizures, improve quality of life, and reduce morbidity and mortality while avoiding the complications of traditional surgery.
“Notably, together with our colleague Dr. Yanrong Zhang at Stanford, we have recently tested the PING approach in two animal models of temporal lobe epilepsy,” added Dr. Lee. “Both of those studies resulted in a substantial reduction or elimination of seizures, underscoring the potential efficacy of PING for treating epilepsies that are not responsive to anti-seizure medications.”
The study was funded by the National Institutes of Health, the Chester Fund, and the Focused Ultrasound Foundation. In addition, one of the contributing authors on the paper, James Woznak, was one of the Foundation’s global scholars.
See the Journal of Neurosurgery >
See the University of Virginia’s Coverage >
See Media Coverage and an Interview with Dr. Lee on WMRA Radio >
