The heat is on at Celsion
Small biotech is making giant strides in heat-mediated targeted drug delivery
 Heat-sensitive nanotechnology is moving closer to dramatically changing cancer therapy. In the near future, this technology will be used to deposit chemotherapy directly onto tumors, minimizing toxic side effects and maximizing treatment efficacy. Though small in size (17 employees), Maryland-based Celsion Corporation is making a huge impact on this important and emerging area of medicine.
Heat-sensitive nanotechnology is moving closer to dramatically changing cancer therapy. In the near future, this technology will be used to deposit chemotherapy directly onto tumors, minimizing toxic side effects and maximizing treatment efficacy. Though small in size (17 employees), Maryland-based Celsion Corporation is making a huge impact on this important and emerging area of medicine.

Founded in 1982 as Cheung Laboratories, Inc., Celsion specializes in developing products for heat-based medical treatments. In its early years, the company produced devices. It turned its attention to drug making in the late 1990’s after licensing a heat-activated liposomal technology from Duke University. That technology serves as the platform for the company’s first investigational nanomedicine, ThermoDox, a liposome-encased form of a potent, widely used cancer drug, doxorubicin.
In the future, Celsion expects to use its liposomal encapsulation technology for other therapeutics and indications.
First investigational drug: ThermoDox
One hundred nanometers in size, ThermoDox liposomes transport doxorubicin intact through a patient’s bloodstream to treatment sites (tumors) that have been heated to the level of mild hyperthermia (40-42 degrees Celsius; 104-107.6 degrees Fahrenheit). When activated by the heat, the liposomes restructure and create channels through which doxorubicin rapidly disperses into surrounding tissue, precisely where needed.
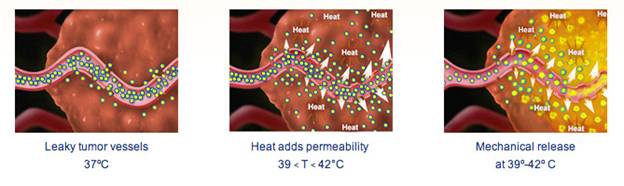
Two clinical trials are now evaluating ThermoDox as a treatment for primary liver cancer (the global 600-patient Phase III HEAT Study) and for recurrent chest wall breast cancer (the stateside 100-patient Phase I/II DIGNITY Study). The HEAT study is using radiofrequency ablation to both activate ThermoDox and destroy liver tumors. Expected to be completed by June 2010, the DIGNITY trial is using a non-ablative mild heat energy to trigger the drug’s release. Celsion expects to file New Drug Applications after completion of each study.
Later this year, the company will begin a randomized Phase II study to evaluate ThermoDox and radiofrequency ablation as a treatment for colorectal liver metastases. Montefiore Medical Center in New York City will be the lead site, and Celsion expects to add at least two other study locations in North America and in the Asia Pacific region. Launch of the new trial follows completion of a Phase I safety study involving 24 patients, 15 of whom had liver metastases from nine primary sites.
Combining MR-guided FUS with ThermoDox
Working in partnership with Royal Philips Electronics (parent company of Philips Healthcare), Celsion has also begun exploring the use of ThermoDox in combination with magnetic resonance-guided high intensity focused ultrasound (FUS) to treat various solid tumor cancers. Researchers at the U.S. National Cancer Institute, Sunnybrook Health Sciences Centre in Toronto and Université de Bordeaux in France have helped establish technical parameters for this combined therapy. Celsion is reported to be in discussions with the FDA regarding the launch of Phase I/II clinical trials to evaluate ThermoDox with MR-guided FUS in treating metastatic bone cancer and pancreatic cancer.
In recent weeks, the Center for Translational Molecular Medicine, a public-private research consortium based in the Netherlands, awarded 6.4 million Euro (approximately $8.7 million U.S.) to Celsion and Philips to develop FUS-mediated ThermoDox therapies for liver tumors and secondary bone tumors. Set to begin in May 2010, the project will be led by the University Medical Center Utrecht in the Netherlands. Also participating are Technical University Eindhoven in the Netherlands and the National Institutes of Health’s Clinical Center in the U.S. As a first step, the group will conduct pre-clinical studies to assess doxorubicin drug delivery and to optimize MR-guided FUS performance in this application. According to Celsion, an Investigational New Drug submission is planned for 2010, following successful completion of the pre-clinical studies.
Written by Ellen C., McKenna
