 The United States Food and Drug Administration (FDA) recently announced that it had approved Profound Medical’s Sonalleve MR-guided High Intensity Focused Ultrasound (MR-HIFU) system for the treatment of osteoid osteoma (OO) in the extremities. This marks the first focused ultrasound regulatory approval that will directly impact pediatric patients, and it is the sixth indication to earn approval in the US.
The United States Food and Drug Administration (FDA) recently announced that it had approved Profound Medical’s Sonalleve MR-guided High Intensity Focused Ultrasound (MR-HIFU) system for the treatment of osteoid osteoma (OO) in the extremities. This marks the first focused ultrasound regulatory approval that will directly impact pediatric patients, and it is the sixth indication to earn approval in the US.
Osteoid osteomas are painful bone tumors that make up about 10 percent of all benign bone tumors. They are most common in patients under the age of 25, particularly adolescents, with males affected three times more often than females. The major symptom is severe pain, but growth of the tumor can also have significant impacts on bone structure and development. When symptoms interfere with activities of daily life, many patients are referred for treatment.
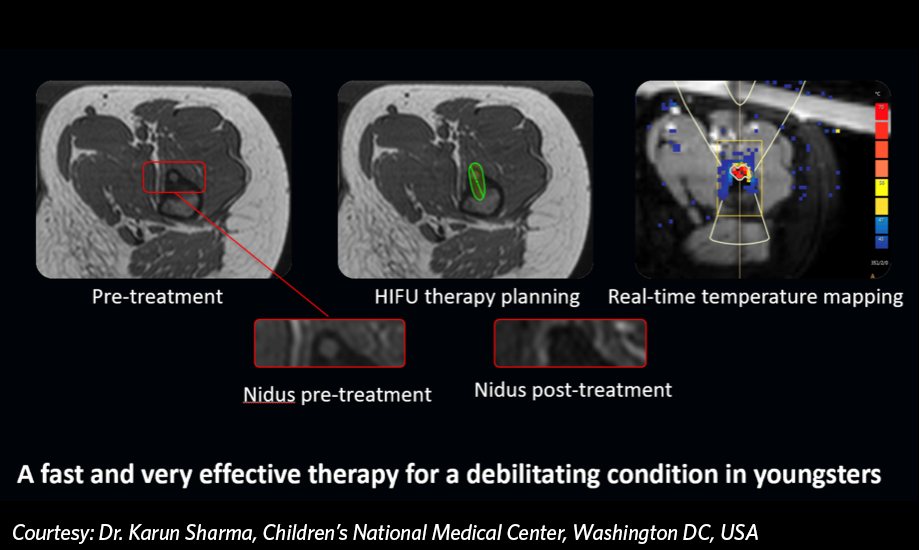
The Sonalleve device was approved under a Humanitarian Device Exemption (“HDE”), reducing the need to demonstrate effectiveness in addition to safety. However, in the release, the FDA noted, “Clinical results support the probable benefit of Sonalleve MR-HIFU system for the ablation of painful osteoid osteoma. Efficacy was evaluated in a study of nine patients treated with MR-HIFU, without technical difficulties or serious adverse events. There was a statistically significant decrease in their pain scores within four weeks of treatment. No pain medication usage was achieved in 8 of 9 patients after four weeks.”
“Obtaining the first regulatory approval for Sonalleve® in the U.S. is a significant milestone for the Company and we are making preparations for the US commercial launch in 2021,” said Arun Menawat, Profound’s CEO and Chairman. “Osteoid osteoma is a benign bone tumor that occurs most often in young children and adolescents. It causes severe pain, especially at night. This noninvasive treatment option is like a ‘Star Trek’ story that destroys the tumor without incision or radiation.”
 “Focused ultrasound is ideal for treating these painful bone tumors,” said Foundation Chairman Neal F. Kassell, MD. “As this condition affects primarily a pediatric population, focused ultrasound offers a noninvasive therapy that avoids harmful radiation and overuse of pain management medication. This ruling marks an important milestone for using the technology in pediatrics.”
“Focused ultrasound is ideal for treating these painful bone tumors,” said Foundation Chairman Neal F. Kassell, MD. “As this condition affects primarily a pediatric population, focused ultrasound offers a noninvasive therapy that avoids harmful radiation and overuse of pain management medication. This ruling marks an important milestone for using the technology in pediatrics.”
More work is underway for OO. The Foundation is supporting an ongoing comparative trial being conducted at the University of California San Francisco and Stanford University. That study is investigating the use of another focused ultrasound device manufactured by Insightec. Another NIH funded clinical trial is also open at Children’s National Hospital and using the Sonalleve device for painful OO treatment in children and young adults.
Focused ultrasound is also FDA approved to treat essential tremor, tremor-dominant Parkinson’s disease, uterine fibroids, pain from bone metastases, and the prostate.
