 Taiwan-based EpiSonica has secured Taiwan Food & Drug Administration (TFDA) clearance of its ArcBlate MR-guided focused ultrasound system for soft tumor ablation of uterine fibroids, adenomyosis, and palliative pain care. ArcBlate can be used in any commercial MRI system.
Taiwan-based EpiSonica has secured Taiwan Food & Drug Administration (TFDA) clearance of its ArcBlate MR-guided focused ultrasound system for soft tumor ablation of uterine fibroids, adenomyosis, and palliative pain care. ArcBlate can be used in any commercial MRI system.
The ArcBlate focused ultrasound system is designed unlike any other on the market. The transducer moves around an ARC structure that is located above the supine patient inside of the MRI scanner. The advantages of this design allow “greater patient comfort, wider coverage, more flexible focusing, shorter treatment time, and a reduced risk of skin burns,” according to CEO Jimmy Chang. ArcBlate can be used inside any existing 1.5T or 3T MRI system, and it is fully automated to execute planned procedures without operator intervention. Besides ArcBlate, EpiSonica is also developing a new breast imaging tomography system.
Q & A with EpiSonica CEO Jimmy Chang
Q. How did you get involved in starting EpiSonica?
I started my career in 3D ultrasound at TomTec Imaging Systems (USA). In 2006, I was involved in the start-up and clinical trials of an ultrasound-guided focused ultrasound (FUS) system in China. Although its potential for FUS was exciting, we realized the limitations associated with single-plane ultrasound guidance and a lack of reliable means to verify protein denaturation during treatment. So, in 2013, when Dr. Hsu Chang at the Taiwan National Health Research Institute (NHRI) asked me to lead a spin-off a government-supported MR-guided FUS project into commercialization, I was attracted by the fresh design, engineering concepts, and the challenge to upgrade the ARC platform to existing MRI systems. I saw the business potential and accepted the position.
Q. Tell us about your company structure: ownership, lead executives, and their roles.
EpiSonica was established with a core Research & Development (R&D) team led by Dr. I. H. Kuo from NHRI. The venture capital came from a group of physicians from the United States and Taiwan, Angels, and local venture funds.
The management team includes seasoned executives in business development, R&D, finance, and regulatory affairs. A clinical advisory board led by Dr. Phil Templeton (retired as Chairman of the University of Maryland Department of Radiology) was formed and recruited experts in Interventional Radiology, Minimally Invasive Gynecology, Oncology, and Breast Surgery.
 Q. In general, what is the current status of your company?
Q. In general, what is the current status of your company?
Thus far EpiSonica has developed two products: – MRgFUS and ABUS (Automated Breast Ultrasound Tomography). Both are ready for commercializing. A third product is being planned to eventually merge the two platforms into a futuristic, non-invasive breast tumor 3D imaging and treatment system. Today we have more than 30 employees (70% R&D) plus a group of technical consultants with various expertise. We have started to receive preliminary customer orders for both products. An international distribution and service network is in place, and it is expanding as we reach out by participating in exhibitions and seminars. We are closing our Series C Funding in November and plan to be profitable by the middle of 2018 before offering public shares on the Taiwan Stock Exchange.
Q. What are the benefits of your technology over other systems on the market?
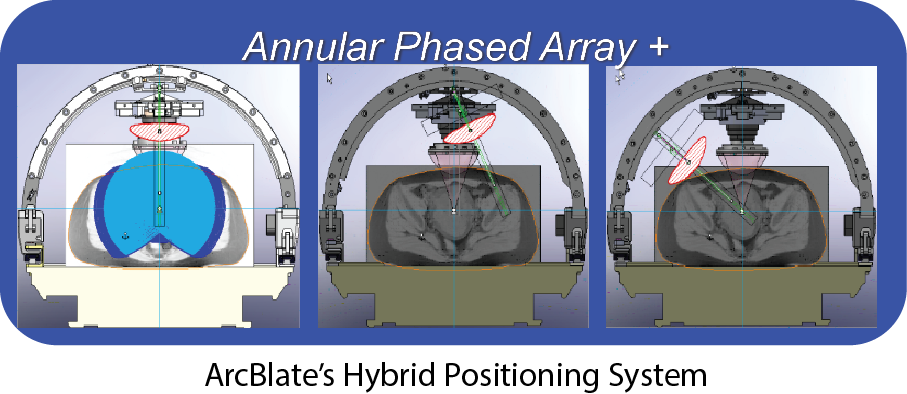 With our patented ArcBlate hybrid positioning technology, the ablation transducer is not hidden inside a water tank situated within the MRI table as seen on other commercially available systems. Our design provides a much wider angle for the ablation path over the patient and allows for more flexible treatment plans and the ability to avoid scars or tissue obstacles (i.e., intestines). Placing the degassed disposable water bag on top of the supine patient’s belly decreases the risk for skin burns. Patients are also more comfortable and can relax in the supine position during treatment. Our clinical trials have suggested the potential for an easier and shorter treatment process that decreases time in an expensive MRI suite. Equally important as the clinical benefits, the ArcBlate upgrade solution provides a simple pathway for both MRI vendors and existing hospital MRI systems to be fitted and transformed at fraction of the cost, potentially making FUS widely accessible to hospitals and their patients.
With our patented ArcBlate hybrid positioning technology, the ablation transducer is not hidden inside a water tank situated within the MRI table as seen on other commercially available systems. Our design provides a much wider angle for the ablation path over the patient and allows for more flexible treatment plans and the ability to avoid scars or tissue obstacles (i.e., intestines). Placing the degassed disposable water bag on top of the supine patient’s belly decreases the risk for skin burns. Patients are also more comfortable and can relax in the supine position during treatment. Our clinical trials have suggested the potential for an easier and shorter treatment process that decreases time in an expensive MRI suite. Equally important as the clinical benefits, the ArcBlate upgrade solution provides a simple pathway for both MRI vendors and existing hospital MRI systems to be fitted and transformed at fraction of the cost, potentially making FUS widely accessible to hospitals and their patients.
Q. How many years has your treatment platform been in development, and what are its origins? Does it have a name?
The project began in 2005 and was initially led by Dr. Hsu Chang at the Taiwan NHRI and supported by government grants. Both in vitro and in vivo animal studies were conducted at NHRI, and 26 international patent applications were filed and granted. After EpiSonica was formed, the company continued R & D efforts with a focus on commercialization. The product platform is called ArcBlate.
Q. What are some of the technical challenges your group has to overcome to develop a fully noninvasive system?
ArcBlate is designed as a FUS platform that can be guided by either MRI or ultrasound. To make it compatible with most existing commercial 1.5T/3T MRI systems, we faced and overcame many technical challenges. ArcBlate’s Hybrid Positioning System employs an ARC structure that rises over the top of the patient’s body while she is laying face up (supine). The Annular Phased Array Transducer is connected to a replaceable water bag that is mobilized by the ARC.
The most notable technical challenge was designing the interference-free, fully automated ARC transducer assembly to work inside of existing MRI systems with no modifications to the MRI. This is a remarkable accomplishment when considering the amount of action that takes place inside the MRI bore and the fact that EpiSonica is an independent company with no support from any of the MRI vendors.
The work-in-progress ultrasound-guided system will feature the same ARC and transducer assembly that is interchangeable between MRI and ultrasound guidance. When guided by ultrasound, the system is no longer restricted with “magnetic interference free” materials, which could lower the cost. Our ultrasound-based thermometry technology is still under development.
Q. What challenges do you have to tackle moving forward?
To fully explore the market potential beyond 1.5T/3T MRgFUS, EpiSonica is interested in pursuing both open MRI guidance as well as ultrasound (US) guidance. The technical challenges for open MRI compatibility are greater than those met for the 1.5T/3T units because most open MRI systems produce less consistent T1 phased data for thermal imaging. The bore space is also much smaller for integrating EpiSonica’s ARC design. The technical challenges associated with USgFUS will be the need to develop reliable real-time monitoring of tissue treatment and 3D guidance to avoid the common criticism that commercial USgFUS systems experience errors in the ablation trajectory planes because they are limited by 2D ultrasound imaging.
Q. Tell us about your clinical studies and the results.
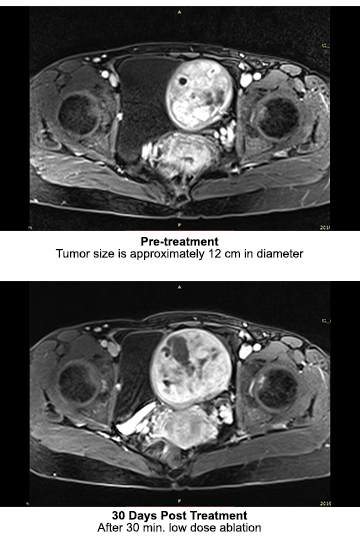 In 2015, the first clinical trial to test safety during uterine fibroid ablation was conducted under Taiwan FDA IRB approval. A total of 6 patients were treated, and the results proved the device to be safe and effective. Neither anesthesia nor sedation was used during the treatments. No side effects were observed, and all patients reported that they were comfortable with no sense of heat or pain during the treatment. Because the trial only tested safety, the total ablation time was limited to a maximum of 10 minutes; however, several patients reported significant symptom relief at their 90-day checkup.
In 2015, the first clinical trial to test safety during uterine fibroid ablation was conducted under Taiwan FDA IRB approval. A total of 6 patients were treated, and the results proved the device to be safe and effective. Neither anesthesia nor sedation was used during the treatments. No side effects were observed, and all patients reported that they were comfortable with no sense of heat or pain during the treatment. Because the trial only tested safety, the total ablation time was limited to a maximum of 10 minutes; however, several patients reported significant symptom relief at their 90-day checkup.
In July 2016, a clinical trial of 10 patients targeted at reliving the symptoms of uterine fibroids began in the department of gynecology at the Chang Gung Memorial Hospital, and on-going trials are in progress. At National Taiwan University Hospital, we are participating in a clinical trial of 50 patients to relieve the symptoms of uterine fibroids and adenomyosis, or for palliative relief of the pain from pancreatic cancer. This study is being jointly conducted by the departments of Interventional Radiology, General Surgery, Gynecology, & Oncology and is currently undergoing the IRB approval process. Further clinical trials in Japan and Korea will be conducted in 2017 with support from EpiSonica ODM and distribution partners.
Q. Have you learned any lessons for watching the experience of the other companies?
As biomedical engineers and entrepreneurs who have developed both USgFUS and MRgFUS systems at length, we at EpiSonica understand the limitations of the FUS technology. Only when we well appreciated these limitations could we make clinical and economic sense of this amazing technology. We observed that when some MRI vendors introduced MRgFUS systems to interventional radiologists for treating uterine fibroids, often the radiologists could not get support from referring gynecologists. When gynecologists expressed interest in operating the focused ultrasound system, they found the commercial systems difficult to use and were uncomfortable managing their patients in the MRI suite. These types of issues inspired us to design a system that is user friendly to non-MRI experts and to create a business model that brings gynecologists and radiologists together.
Q. Is your system approved for commercial use in any markets? If so, how is it being used in these markets?
On September 26, 2016, the Taiwan FDA cleared ArcBlate for commercialization for the indication of soft tissue tumor ablation. EpiSonica will install ArcBlate systems in several top medical centers in 2017, and these will initially target uterine fibroid and adenomyosis treatment. After clinical benefits for pain palliation are developed, additional services for pain management will be planned.
Past Coverage of Company
November 2015 New Focused Ultrasound Companies Formed in Taiwan
