Key Points
- Researchers in Taiwan published data from a small pilot clinical trial testing the use of focused ultrasound neuromodulation in patients with drug-resistant epilepsy.
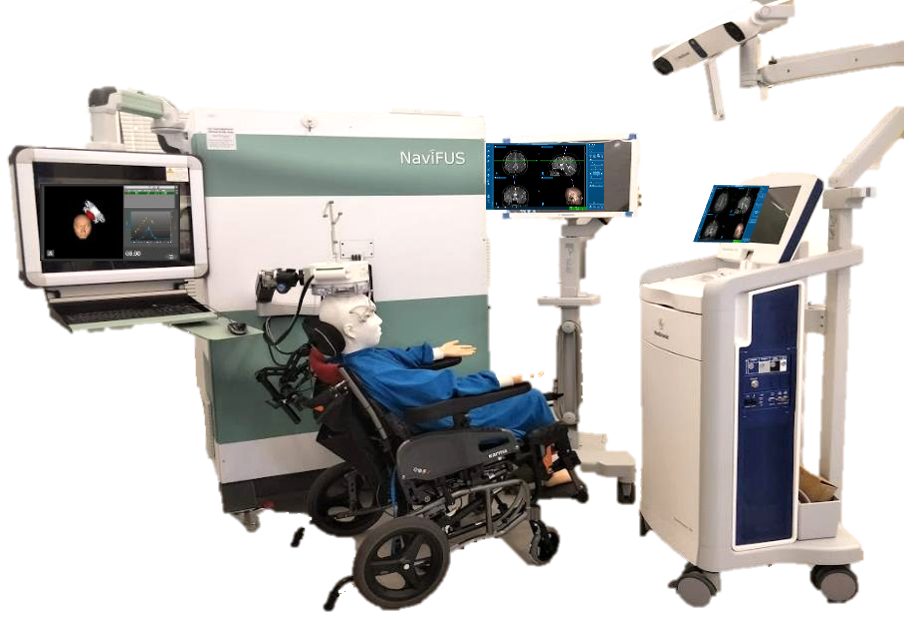
- Participants received low-intensity focused ultrasound in the seizure origination zone from the NaviFUS neuronavigation-guided system.
 Pilot Study of Focused Ultrasound for Drug-Resistant Epilepsy
Pilot Study of Focused Ultrasound for Drug-Resistant Epilepsy
Researchers in Taiwan recently published the data from a small pilot clinical trial testing the safety and efficacy of using focused ultrasound to provide neuromodulation for patients with drug-resistant epilepsy.
The pilot trial enrolled six patients undergoing stereo-electroencephalography (SEEG), a minimally invasive procedure used to locate the area of the brain where seizures originate. After meeting the enrollment criteria and completing the SEEG procedure, participants received low-intensity focused ultrasound in the seizure origination zone from the NaviFUS neuronavigation-guided system. SEEG recordings were obtained during neuromodulation and for three days after treatment. The researchers then collected data on seizures, brain activity related to epilepsy, and adverse events. The neuronavigation was provided by the Medtronic stealthStation S7 system, which displays the treatment plan in real time throughout the process.
Low-intensity focused ultrasound was safely delivered with no significant adverse events, and the monitoring of the neuromodulation effect showed promise for the procedure. Seizure frequency decreased in two patients during the three-day follow-up period, but one patient experienced an increase in the frequency of subclinical seizures. One patient reported scalp heating during the procedure, and one patient developed a temporary memory impairment.
The researchers proposed continuing the work with a larger group of patients and testing an additional range of sonication parameters for seizure control.
NaviFUS Corporation provided funding and technical support for the investigators but did not direct study design, patient enrollment, patient outcome analysis, or data interpretation. All coinvestigators conducted the study and wrote and submitted the manuscript for publication without interference from the company. Additional funding was provided by the Taiwan Ministry of Science and Technology (107-2314-B-075-059-MY3 and 109-2314-B-075-053) and National Health Research Institutes (NHRI-EX109-10905NI, NHRI-EX110-11006NC).
