EDAP TMS is a French company specialized in focused ultrasound treatment of the prostate. In 2015, the US Food and Drug Administration approved their Ablatherm® Robotic HIFU device, and their Focal One® device earned approval earlier this year. We recently interviewed three members of the company’s leadership: Emmanuel Blanc, Director of Research & Development; Vincent Mortureux, HIFU Product Manager; and Maude Pavageau, Communications Manager.
 How was the company started?
How was the company started?
Founded in 1979, EDAP TMS is committed to introducing new and innovative technologies that provide a minimally invasive approach for treating medical disorders. The company started by manufacturing ultrasound scanners and then moved into lithotripsy by developing the first medical device offering a completely noninvasive therapy to treat renal stones. EDAP TMS’ proprietary technologies for focused ultrasound and electroconductive shock waves have yielded new minimally invasive procedures for the treatment of localized prostate cancer and urinary tract stones, following its design philosophy to develop safe and effective minimally invasive devices for the benefit of the patient. The company is further committed to developing treatments for more disorders in many organs.
Who is the Chief Executive of EDAP TMS?
Marc Oczachowski is our Chief Executive Officer. He joined the company in May 1997 as an Area Sales Manager based in Lyon, France. From 2001 to 2004, he held management positions including General Manager of EDAP Technomed Malaysia. He was appointed Chief Operating Officer in November 2004 and became Chief Executive Officer in March 2007.
Tell us about your company structure: ownership, lead executives, and their roles.
A global company from Research & Development to Customer Care, EDAP TMS is headed by our Chief Executive Officer and managed by department heads in Research & Development, Industrial Operations, Quality Affairs, Regulatory Affairs, Marketing, Commercial, Sales Administration, Application Services, Customer Care, Human Resources, and Investor Relations. EDAP TMS is also active worldwide through a network of subsidiaries (5), corporate offices (3), and various local distributors.
In general, what is the current status of your company?
With approximately 200 employees worldwide, including 140 at headquarters, EDAP TMS is a small-to-medium-sized enterprise that maintains a start-up spirit.
How many years has your focused ultrasound treatment platform been in development, and what are its origins?
With regard to high-intensity focused ultrasound (HIFU) for prostate cancer, we needed 10 years to get the first approval and 15 additional years to get reimbursement. We have developed two generations of medical devices. The first one, Ablatherm® HIFU, offers precise local treatment in a single session. The second, Focal One®, is the first medical device dedicated to focal therapy of prostate cancer. It combines the latest technologies in diagnosis, therapy, and treatment control, and the treatment specifically targets the area to be treated.

What are some of the technical challenges that your group had to overcome to develop a fully noninvasive system?
One of the main challenges was managing the complex interactions between ultrasound and tissue: the many physical effects needed time to be controlled. But conversely, learning these tissue interactions has also opened the door to using these mechanisms of ultrasound in many different clinical applications (e.g., ablation, stimulation, drug release, cellular permeability). Another big challenge was making a complex technology accessible to any user while guaranteeing safe, effective, and reproducible procedures.
What challenges do you have to tackle moving forward?
Our big challenge to tackle moving forward is reimbursement of the procedure by local health authorities. This is the key to accessing the market and allowing the procedure to be positioned as a reference option in the therapeutic arsenal for localized prostate cancer.
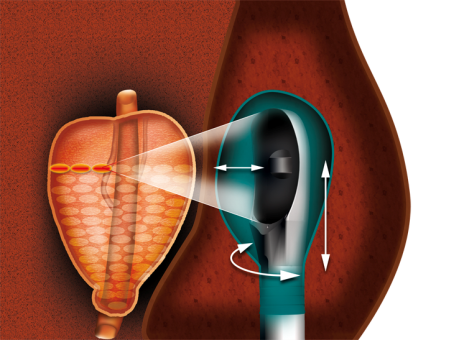 Tell us about your clinical studies and the results.
Tell us about your clinical studies and the results.
We have conducted many single arm clinical studies to prove safety and efficacy of HIFU for the treatment of prostate cancer. It is difficult to design and operate comparative studies (methodology, patient recruitment), but unfortunately these are the studies that sustain reimbursement projects.
What are the benefits of your technology over other companies?
Our products include some unique features that directly benefit the patient and surgeon. First, our patented Dynamic Focusing technology allows electronic displacement of the focal point, which enables the ability to do conformational treatment. This means that the surgeon can plan a treatment with enhanced precision around the target, maximizing the energy in the target while preserving the surrounding tissues, thus reducing morbidity for the patient.
We also developed a strong experience in fully robotized medical procedures. From the planning of the treatment to the execution of the treatment plan, including real time monitoring and associated safety features, the device allows for a completely software-controlled procedure.
Have you learned any lessons by watching the experience of the other companies?
We are all confronted with the complexity of transforming technological concepts to marketed medical products, and the Focused Ultrasound Foundation has a unique and valuable perspective of this across the field. This insight is something that we benefit from.
Do you partner with other companies?
Yes, we partner with the ultrasound scanner and transducer manufacturers whose products are integrated into our devices.
Is your system approved for commercial use in any markets? If so, how is it being used in these markets?
Our HIFU devices are approved worldwide except in Japan. Focal One® was approved in the US in June 2018.
Which health conditions or diseases is your HIFU technology currently used for?
Our HIFU devices are intended to treat localized prostate cancer via global, partial, or focal treatment and we are working hard to address this technology to many more disorders, including pancreatic cancers, endometriosis, and more.
EDAP has a lithotripsy business. What technology do these devices use? What is ESWL mobile services?
Besides HIFU, EDAP offers extracorporeal shockwave lithotripsy (ESWL) with the Sonolith® range of devices. These devices use electroconductive technology, which is ultrasound waves focused on the target (kidney stones). ESWL mobile services is an “a-la-carte” service that allows hospitals to rent a Sonolith® lithotripter for a day or more to treat patients scheduled for ESWL treatment. Designed for hospitals with light ESWL patient-load, Sonolith® Mobile Service offers great flexibility and involves no prior investment or long-term commitment.
