Professor Gail ter Haar, PhD, from the Institute of Cancer Research (ICR), London, presented the following “who’s who” in the development of focused ultrasound in a December 10, 2020, “History of Focused Ultrasound” webinar that was part of the International Society of Therapeutic Ultrasound’s “ISTU On-Air” series.
Professor ter Haar is a founding member of the International Society of Therapeutic Ultrasound and served as its first president from 2001 to 2006. She’s a passionate educator, having supervised 28 PhDs, with another five still in progress, and has also supervised four MDs and eight master’s degrees. Professor ter Haar has directed a research team at ICR in Sutton for more than 30 years. She has provided more than 20 invited plenary lectures and more than 200 invited lectures. She is the deputy editor of the journal “Ultrasound in Medicine and Biology.” She has published 229 peer reviewed manuscripts, edited six books, and written an astounding 34 book chapters, all resulting in a Scopus h-index of 54.
The subject of this webinar is the history of focused ultrasound – more specifically, high-intensity focused ultrasound (HIFU).
Disclaimers
First, a disclaimer. There is insufficient time to discuss the entire history, so there will not be much detail about the history of HIFU for prostate, uterine fibroids, interstitial devices, cardiac devices, or intraoperative devices. Nor will there be much about the history of immune stimulation using ultrasound, histotripsy, or combination treatments. Another disclaimer: I am a physicist; therefore, this is a physicist’s view of the history of HIFU, not a clinician’s view.
The Enthusogram
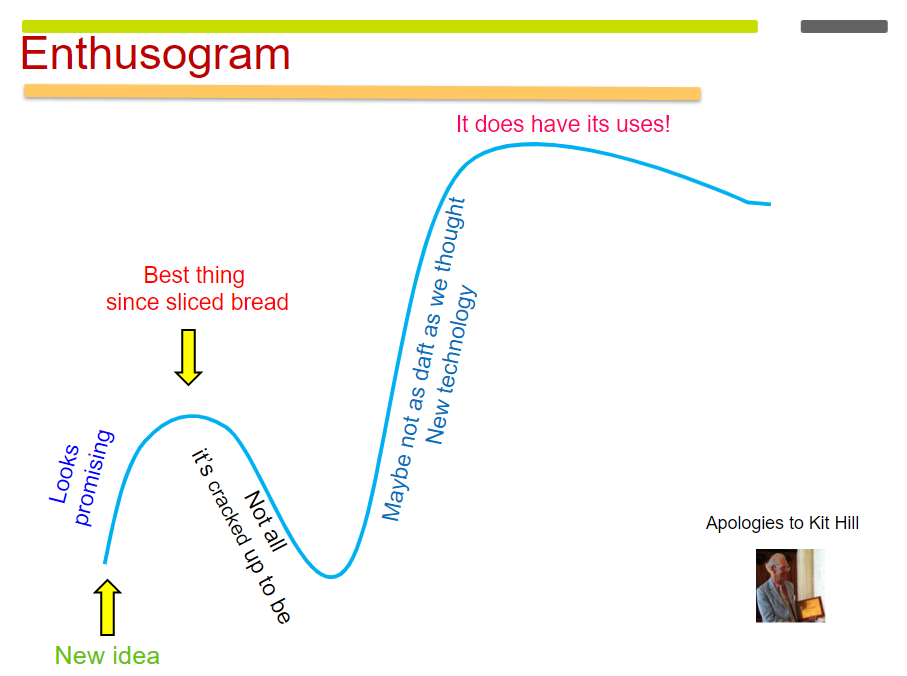
If it is used appropriately, it begins to take off, and we can see that with the development of HIFU. In the early 1950s, there were some brain studies using HIFU. And eventually people started to ask, “What can we use these high-intensity focused beams for other than the brain work?” It looked promising, but perhaps other techniques might be easier. For example, if you’re doing things in the eye, maybe it’s easier to use a laser. If you want to treat Parkinson’s disease, maybe it’s better to use L-dopa.
Enthusiasm for HIFU died away again. But then people returned to the literature – I was one of them – and thought, “Well, maybe we should look at this again. Radiology has caught up, we can make an image, and we can target much better.” Ultrasound and magnetic resonance imaging (MRI) were getting more and more sophisticated. And then there was the recognition that HIFU does have its uses. Now we are on the upward arc of the Enthusogram again.
Number of Publications Over Time
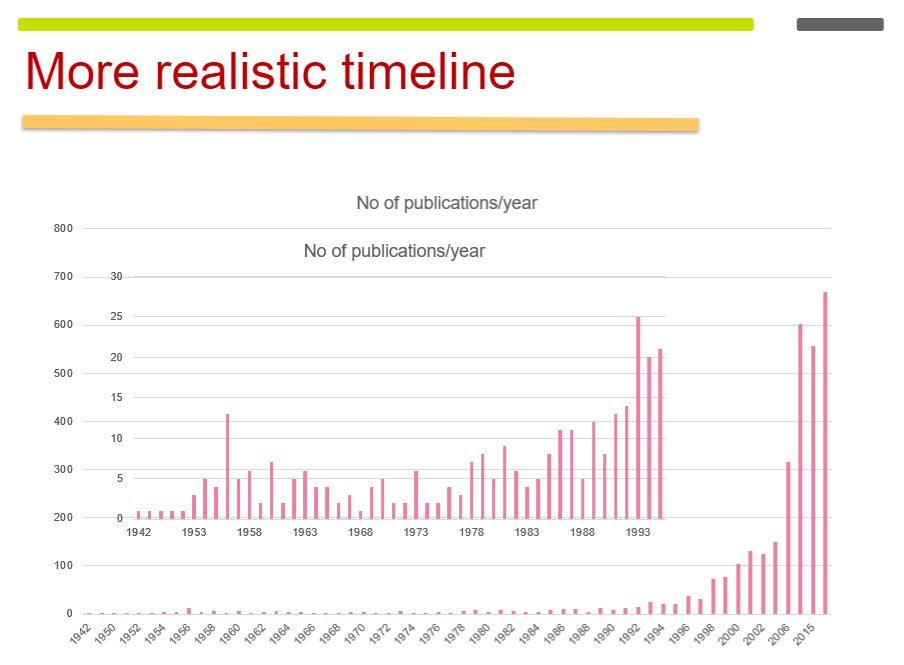
Experimental Beginnings
 The first reported bioeffects of ultrasound were in a beautifully written 1927 paper published by Professor Wood and Mr. Loomis (right). Referring to putting small animals into an ultrasound field, they wrote: “Mice are less sensitive, a 20-minute exposure not resulting in death. Though at the end of the treatment the animal was barely able to move, the recovery was fairly rapid. The biologists inform us, however, that the blood count of a mouse is affected by fear, the corpuscles hiding in the liver until the danger is over!” It’s beautiful writing – we don’t write like that now.
The first reported bioeffects of ultrasound were in a beautifully written 1927 paper published by Professor Wood and Mr. Loomis (right). Referring to putting small animals into an ultrasound field, they wrote: “Mice are less sensitive, a 20-minute exposure not resulting in death. Though at the end of the treatment the animal was barely able to move, the recovery was fairly rapid. The biologists inform us, however, that the blood count of a mouse is affected by fear, the corpuscles hiding in the liver until the danger is over!” It’s beautiful writing – we don’t write like that now.
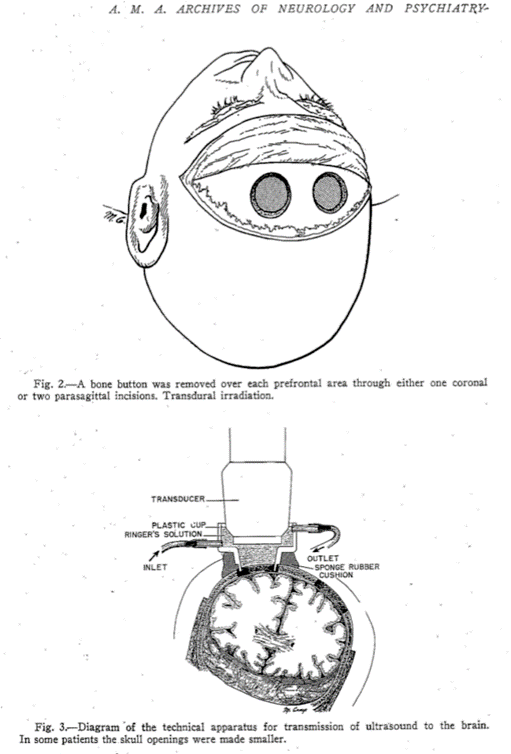
The beginnings of HIFU involved studies in the brain. Early attempts to create lesions in the brain were largely unsuccessful. Scalp damage and skin burns were common, and they found that it was necessary to remove a skull bone flap to access the brain.
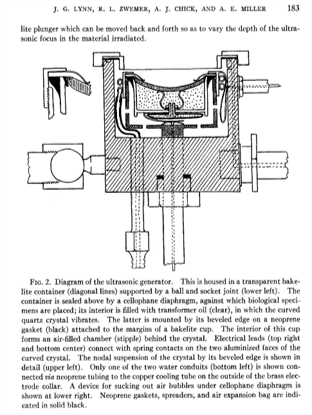
I particularly liked their ideas for the future, some of which could be included in a grant that we are writing today. They said that “the application of treatment at maximum output intensity for minimum period of time was necessary to produce the focal changes.” That makes a lot of sense – if you want to reduce surface heating. And they suggest the “use of a mosaic of 4 to 6 two-inch curved crystals” as the ultrasound source. This is an array – and we’re all using those now. They drew the following conclusions: “In animals, focused ultrasound of high intensity produced local cerebral changes as inferred from behavior disabilities and as demonstrated at autopsy. This local brain effect was achieved through intervening scalp, skull, and meninges. The resulting behavior disabilities disappeared in 2 to 16 hours.” They went on to emphasize the need to improve: “the generation and application of the focused ultrasound beam so that it would be possible to increase still further the focal effects in the brain, and the corresponding decrease or elimination of complicating surface injury.” They knew that they could achieve changes in the brain, and they knew what they wanted to do to make it better. Two years later, in 1944, they did this by lifting a skull flap.
The Fry Brothers
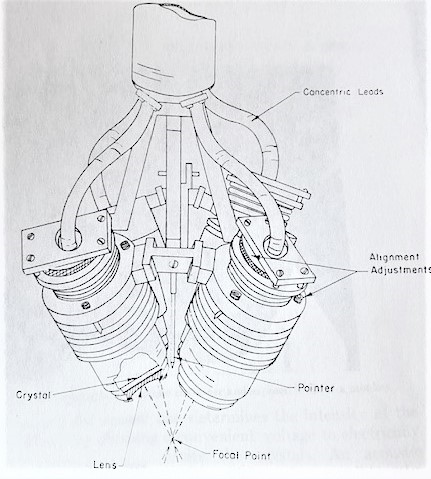
The Fry brothers used a four-transducer system with planoconcave lenses on the front that were aligned to be confocal. They described it in a 1960 paper, which includes a photograph of the transducer and a cross section of the transducer itself. They suggested that they wanted to go on to use a reflector focusing irradiator with a single irradiating head. They filed patents for both the transducer and the coupling device. The coupling device was a bowl with a hole in the bottom to allow the sound through.
We often worry about the fact that our systems are quite large, because we would like to have smaller, more easily transportable systems. The Fry brothers’ system was so large that it occupied two rooms. The transducer came down through the ceiling with all the electronics in the room above. The carriage unit for supporting and moving the irradiators was in the top room, and the treatments took place in the lower room. They were able to make lesions as well as we can now but targeting was much more difficult for them, because they did not have the sophistication of modern imaging techniques.
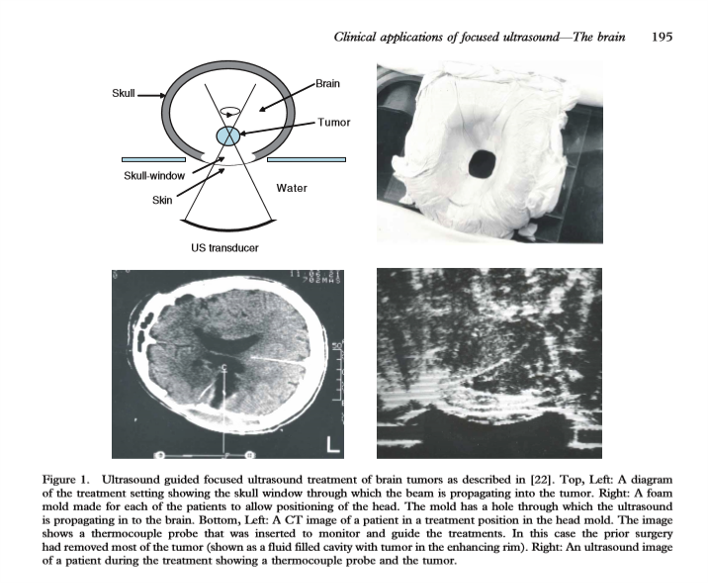
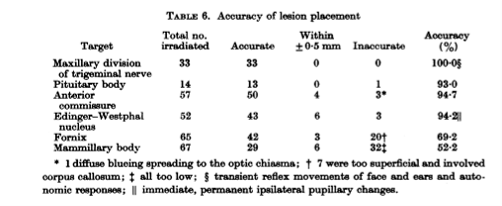
They worked with Robert Heimburger (the first neurosurgeon to use a focused ultrasound device to treat brain cancer, under ultrasound guidance in 1968) to do some human exposures. To do this, they had to create a skull window. The image (below) shows the ultrasound transducer and the brain tumor that they were targeting. It also shows the coupling used in this case along with the image they were trying to work with. Early ultrasound images were hard to interpret.
Other Early Pioneers
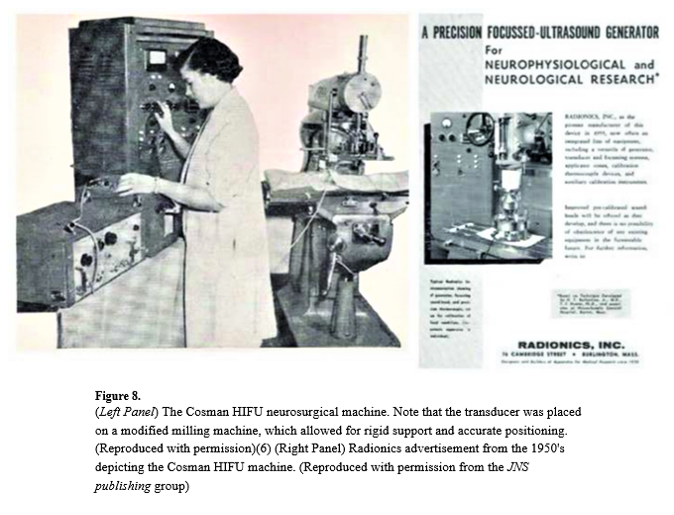
Thomas Ballantine and his colleagues at Massachusetts General Hospital used an ultrasound transducer mounted on a system called the Cosman HIFU neurosurgical machine to perform human treatments in the brain. The caption notes that the transducer was placed on a modified milling machine for rigid support and accurate positioning. I am particularly fond of this, because when I started my PhD many years ago at Guys Hospital, we also had a milling machine that we used to move the ultrasound transducer. John Pond was doing some work with Mary Dyson then – looking at histology, and he also used a milling machine to create larger lesions.
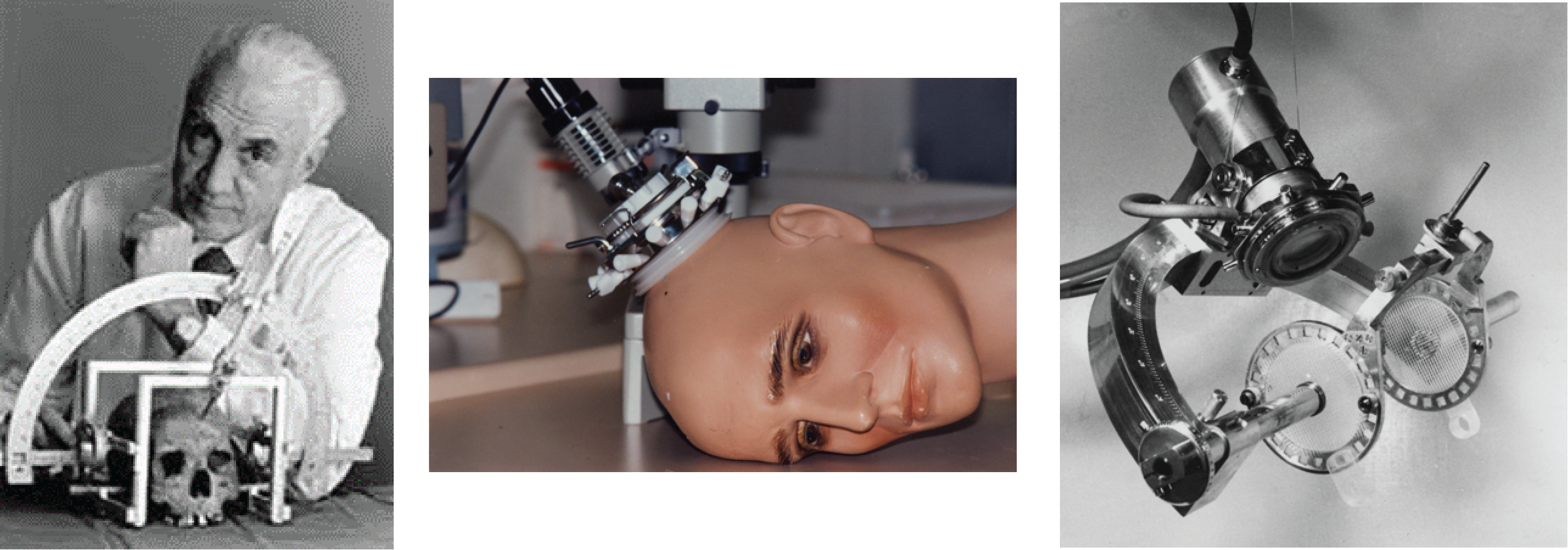
In Sweden, Lars Leksell (pictured below with his stereotactic frame) was working to develop HIFU for treating psychiatric disorders, something that we are only now coming back to. His team successfully produced a system for creating periventricular lesions and developed two transducers between 1949 and 1950. Leksell was frustrated by both the need for a craniotomy and by the poor quality of ultrasound imaging at the time. Therefore, he turned toward developing the brain-based stereotactic radiosurgery for which he made his name. He was pivotal in inventing the Gamma Knife in 1967.

Early Clinical Trials
Let’s look at the results from the early clinical trials. The Fry brothers were treating Parkinson’s disease. They treated 50 patients, and they said that symptoms were eliminated, but it stopped there. There were no further reports and no further clinical trials reported. Also, for Parkinson’s disease, the drug L-dopa was arriving. It is easier to administer a drug than to open the skull and do a brain treatment.
Ballantine was treating painful subcutaneous neuromata. He achieved complete pain relief in seven patients, but again, there are no further reports. These findings were promising, but they were not followed up – probably because of the problems of raising a scalp flap and the limited imaging that was available at that time. The prevailing thought became, “We need to be able to get through the skull. We want to avoid having to make a window in the skull.” And the idea was to use MR for imaging and phased-array transducers – that is, phase correction techniques.
Phased Array Transducers
Two groups began using phased array transducers. Kullervo Hynynen in Boston started working on this with Insightec. He published a paper on the clinical applications of focused ultrasound in the brain – showing his multi-element transducer and how he could get to the brain using phase correction techniques.
At basically the same time in Paris, Mickael Tanter and Jean-Francois Aubry were looking at time reversal to create phase correction. I’m not going to delve too deeply into the physics of time reversal, because many readers may already know it, but basically, you fire an ultrasound beam through the skull to see how it’s been distorted by the skull. Next you correct it, and then you fire the corrected beam back into the skull, and you get your focus back. Tanter and Aubry’s random array transducer is pictured below. Aubry worked on his PhD using this model. The image below at right shows the pressure plot with the uncorrected beam next to the beam corrected with time reversal.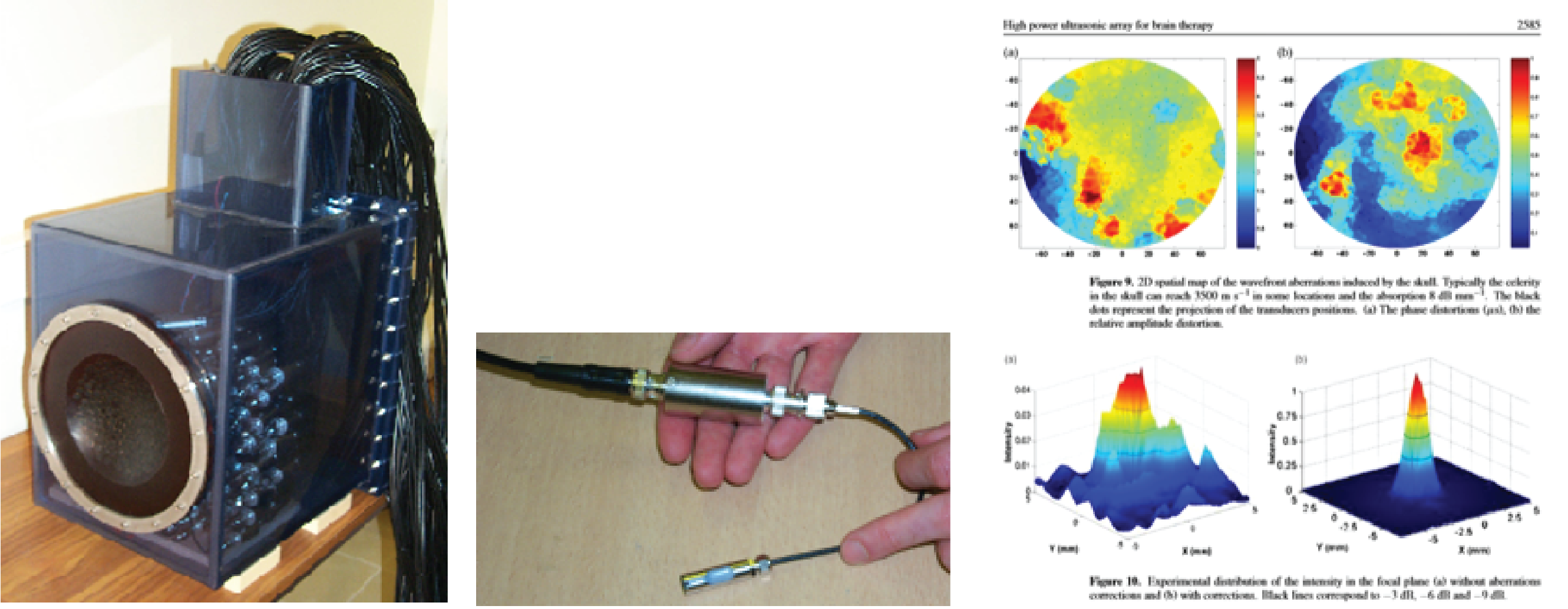
Clinical Devices
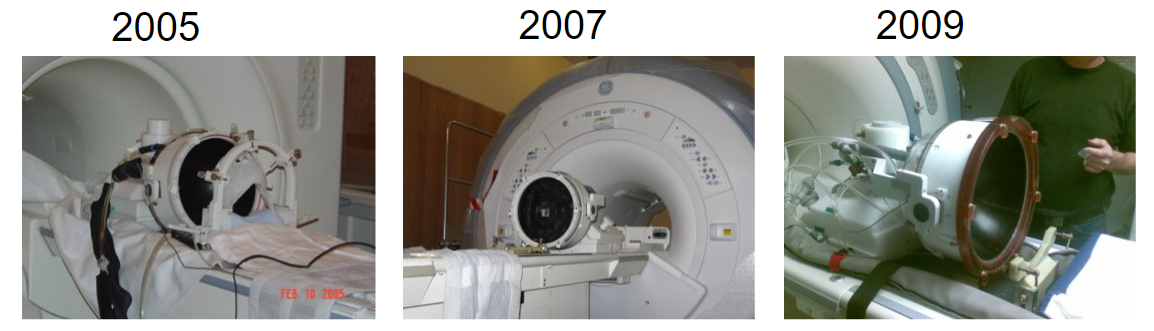
And now brain treatments are performed with a clinical device – an MR-guided system called the Exablate Neuro (below), which is successfully being used for the treatment of essential tremor and other brain disorders. The pictures below from 2005, 2007, and 2009 are courtesy of Kobi Vortman from Insightec.
The Exablate Neuro has been developed and refined over the years as brain applications have become one of the major uses of HIFU. It’s very exciting. It is also interesting and fitting that this was one of the first applications from the 1950s that has now come full circle to be one of the most common applications of HIFU.
HIFU Brain Applications
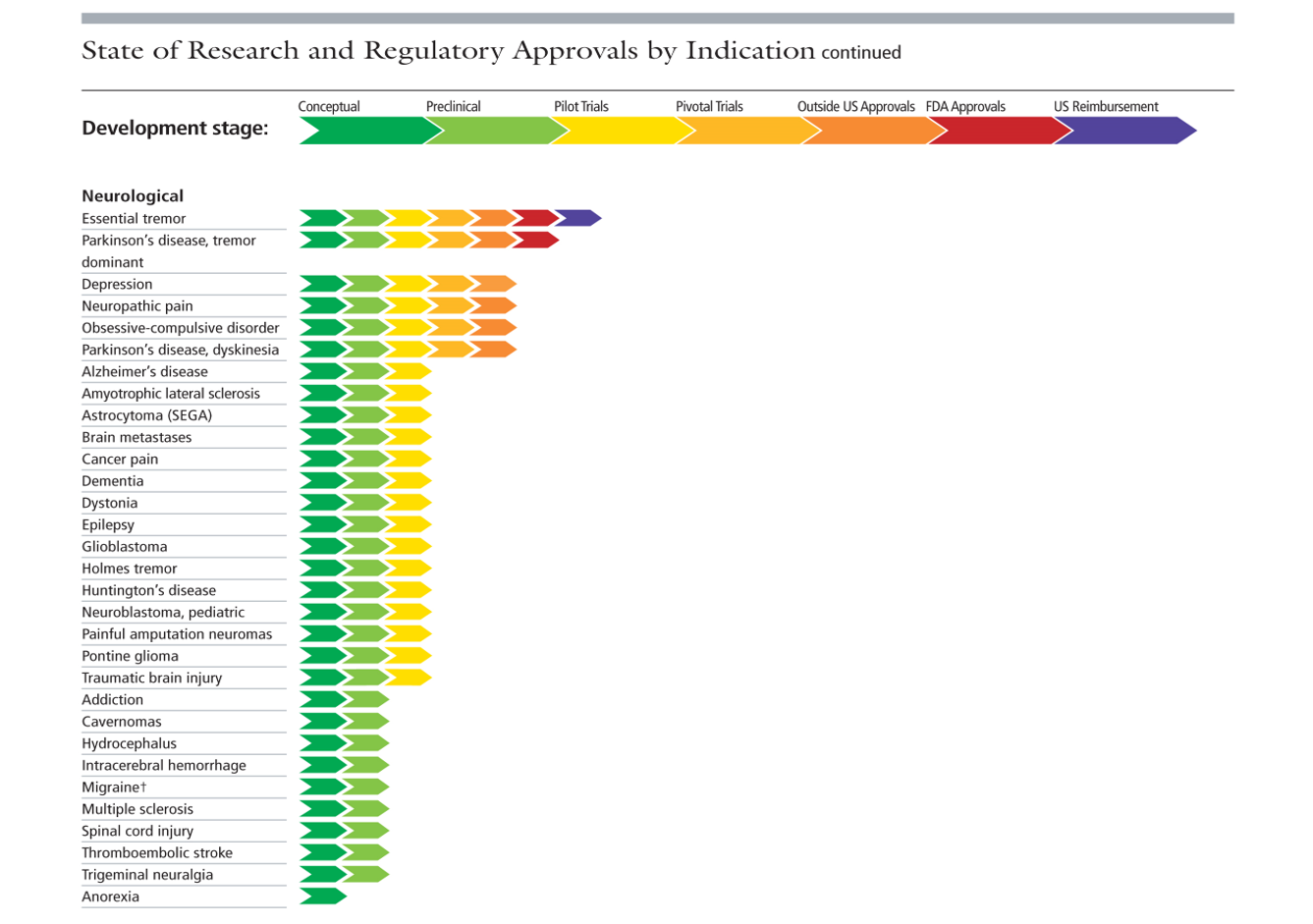
What are we doing now? Well, you only need visit the Focused Ultrasound Foundation website to see the huge range of current brain applications. We’ve come a long way over these past 70 years.
HIFU Eye and Ear Applications

Next, I’d like to mention ear specialist Jack Angell-James, who was working in Bristol. He was collaborating with Professor Peter Wells, who is a well-known ultrasound physicist and the first editor of Ultrasound in Medicine and Biology. They wanted to see if they could treat Ménière’s disease, an unpleasant condition of the inner ear that causes dizziness and tinnitus. Angell-James and Wells used HIFU to selectively irradiate the vestibular portion of the inner ear using a narrow beam of ultrasound to expose the bony labyrinth. They found that this worked quite well. It was also being used by Michele Arslan in Padua, Italy.There are not many publications on this, even though it seemed to have worked, and I’m not quite sure why it disappeared.
HIFU for Cancer
Many people have heard of Nostradamus, a 16th Century French philosopher who wrote predictions. It is interesting that one of these predictions said that sound waves would kill cancer between 1992 and 1998. The first reference I can find to using ultrasound to treat cancer was by the Hungarian, Szent-György, who published in Nature in 1933. He said that the effect of ultrasound radiation on Ehrlich’s carcinoma had been studied by B. Gözsi and was found to have no specific effect on the tumour, so it was a negative finding. But who knows what intensities they were using?
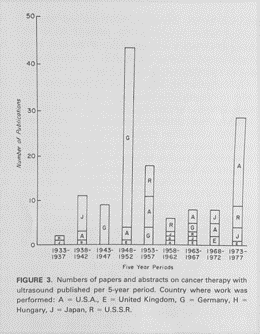 Fred Kremkau from Wake Forest University published a fascinating early review on the use of ultrasound for cancer therapy. His published graph shows the number of papers and abstracts on cancer therapy published between 1933 and 1977 with letters indicating the country of origin. Germany was prolific in publishing about ultrasound and cancer. The graph also includes papers from the United Kingdom, the United States, Hungary, Japan, and Russia.
Fred Kremkau from Wake Forest University published a fascinating early review on the use of ultrasound for cancer therapy. His published graph shows the number of papers and abstracts on cancer therapy published between 1933 and 1977 with letters indicating the country of origin. Germany was prolific in publishing about ultrasound and cancer. The graph also includes papers from the United Kingdom, the United States, Hungary, Japan, and Russia.
In 1949, there was a congress on ultrasound and medicine in Erlangen, Germany, where a lot of papers were presented, some claiming success while others did not. Unfortunately, the conference concluded with “the Erlangen Resolution,” which stated that ultrasound was not suitable for cancer therapy and its clinical use should be discontinued. In 1951, Raimar Pohlman analyzed 133 clinical case reports from the years leading up to 1949 found that 17% showed an improvement, 76% showed no change, and 7% worsened, which might explain why the Erlangen Resolution was issued. It might also be an example of how an excess of enthusiasm, from our Enthusogram earlier, died out. This was the “best thing since sliced bread” part of the enthusiasm parabola — but this wasn’t HIFU – these were low intensities of ultrasound.
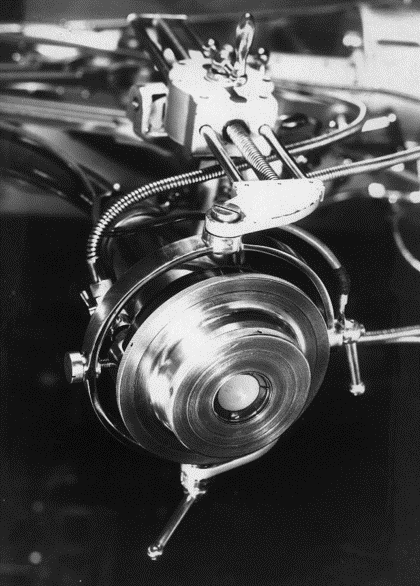 The first real HIFU publications for cancer came from an amazing Russian gentleman, Professor A. K. Burov. Burov was not only a scientist – he was also an architect. He published work on the ultrasound treatment of malignant tumors and described his high-intensity sources. He used unfocused beams at 1.5 megaHertz, with 200 to 500 watts per square centimeter in a pulsed regime (see transducer, pictured). He conducted beam mapping experiments by melting and boiling a plexiglass plate in water. By exposing a Brown-Pearce tumor, which is a tumor that is transplanted into a rabbit testicle, Burov saw a good response in 40% to 80% of tumor cases within several months. The resulting scar was often calcified, but he also observed a clearly pronounced immune response. Burov was one of the earliest to report such a response. Burov’s team also performed clinical trials and melanoma treatments in humans. They treated 10 men in the terminal stage of melanoma, finding that the melanoma completely disappeared in some cases. They reported, “In some cases, positive results were not obtained (several patients died of severe concomitant diseases),” which is expected in a clinical trial, as they often enroll very sick patients for whom there is no other treatment. However, they did see complete resolution of melanoma in some patients.
The first real HIFU publications for cancer came from an amazing Russian gentleman, Professor A. K. Burov. Burov was not only a scientist – he was also an architect. He published work on the ultrasound treatment of malignant tumors and described his high-intensity sources. He used unfocused beams at 1.5 megaHertz, with 200 to 500 watts per square centimeter in a pulsed regime (see transducer, pictured). He conducted beam mapping experiments by melting and boiling a plexiglass plate in water. By exposing a Brown-Pearce tumor, which is a tumor that is transplanted into a rabbit testicle, Burov saw a good response in 40% to 80% of tumor cases within several months. The resulting scar was often calcified, but he also observed a clearly pronounced immune response. Burov was one of the earliest to report such a response. Burov’s team also performed clinical trials and melanoma treatments in humans. They treated 10 men in the terminal stage of melanoma, finding that the melanoma completely disappeared in some cases. They reported, “In some cases, positive results were not obtained (several patients died of severe concomitant diseases),” which is expected in a clinical trial, as they often enroll very sick patients for whom there is no other treatment. However, they did see complete resolution of melanoma in some patients.
If we move on to the 1990s, Guy Vallancien in Paris modified a lithotripter to produce a device called a pyrotherm for treating bladder cancer. He treated 20 patients with bladder tumors under ultrasound guidance. His multi-element transducer worked at one megaHertz with intensities above 10,000 watts per square centimeter. In 20 patients, 75% had normal urinary cytology at a month, and 67% had no recurrence at a year, which is exciting for an early clinical trial. He published his work in various places, including Urology.
Image Guidance
We can selectively destroy tissue with HIFU. Since, if we turn up the intensity, we can achieve instantaneous cell death, it is important that the focus is in the right place. We therefore need to be able to do accurate treatment planning, targeting, monitoring, and follow up. We need excellent imaging. There are rival guidance techniques, as we all are well-aware, including ultrasound imaging and MRI. Each has its advantages and disadvantages.
- For ultrasound, the imaging and therapy beams travel in the same path, producing real-time, high spatial resolution imaging and monitoring of tissues. Ultrasound devices are compact, allowing imaging and therapy to be placed into one hand.
 MRI provides clear anatomical imaging — even through bone, which is desirable for brain treatments. MRI provides temperature mapping whereas ultrasound guidance relies on changes in echogenicity to indicate tissue changes.
MRI provides clear anatomical imaging — even through bone, which is desirable for brain treatments. MRI provides temperature mapping whereas ultrasound guidance relies on changes in echogenicity to indicate tissue changes.
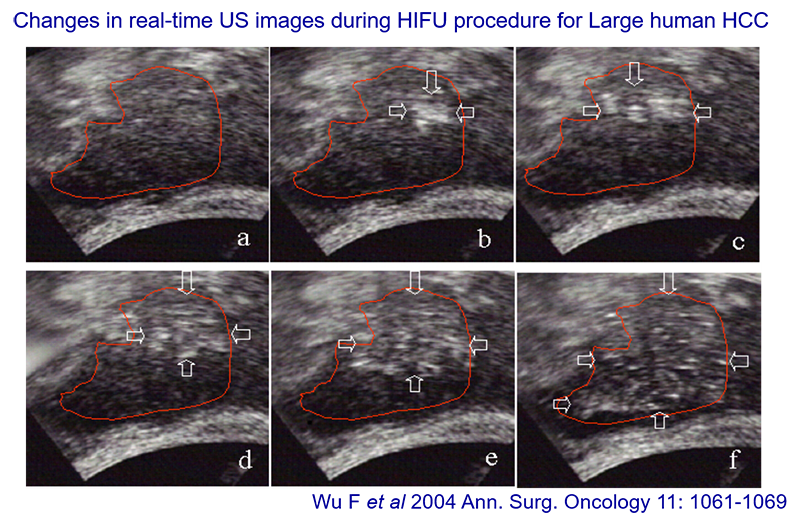
These ultrasound images of a large hepatocellular carcinoma are from a 2004 publication, courtesy of Feng Wu. The area to be treated is in box A. The treatment region is treated point by point, focal point by focal point, or focal volume by focal volume, starting at the back if possible, because the ablation causes changes to the tissue. The difference between the first and last boxes shows the echogenicity changes. The bubbles (bright spots) show the ablation, which is how ultrasound guidance works.
MR guidance relies on temperature mapping using proton resonance frequency shift sequences. The image below shows the displays from two different commonly used MR-guided systems. Temperature mapping allows a treatment to be visualized. The righthand image shows a bone treatment from the Royal Marsden Hospital using the Sonalleve device. This shows successful heating of the surface of the bone to provide pain relief. The temperature scale is shown on the image, and it can be used to calculate thermal dose. After achieving the required thermal dose of 240J, the color change indicates that the treatment is complete.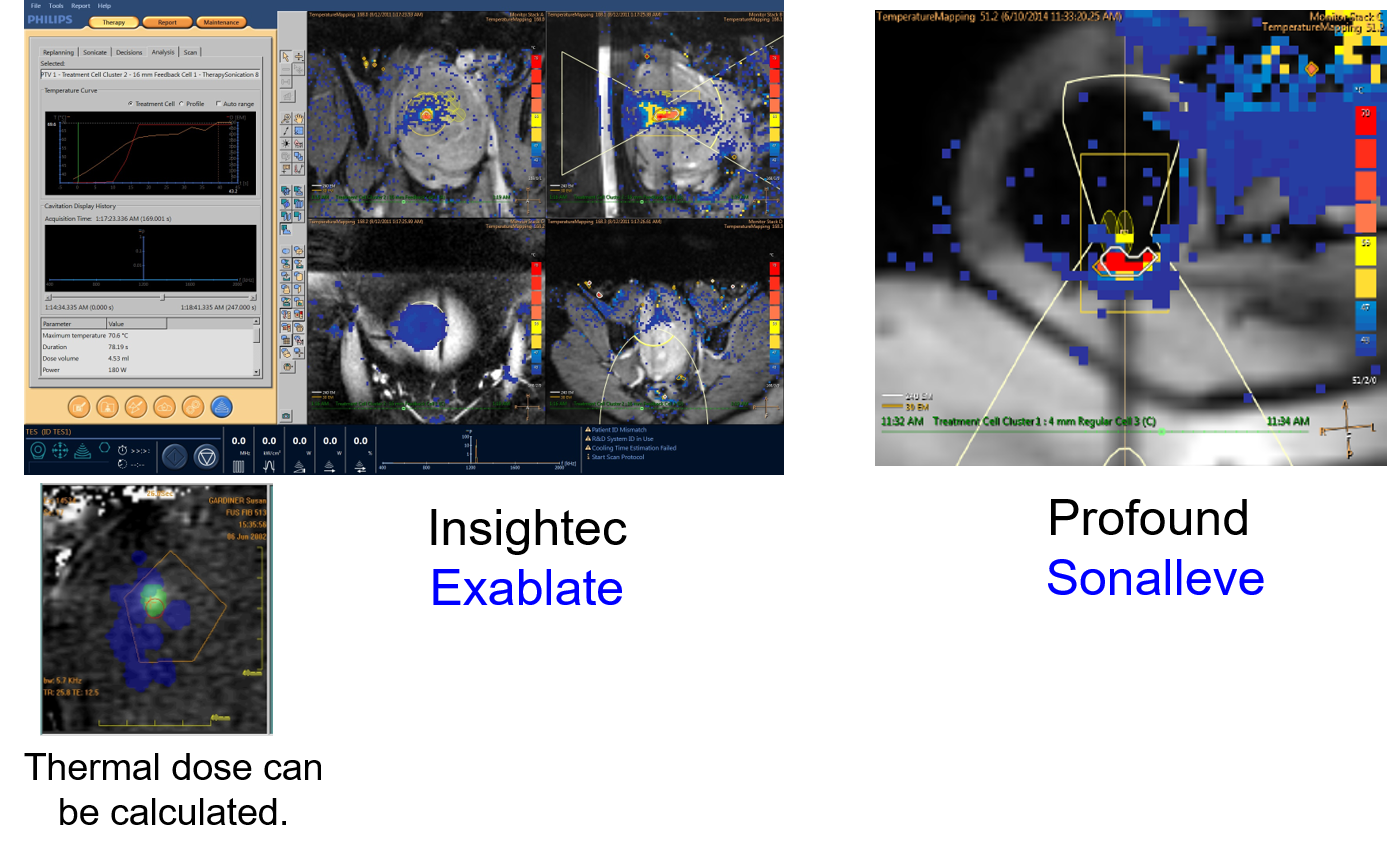
 Early Devices for Clinical Trials
Early Devices for Clinical Trials
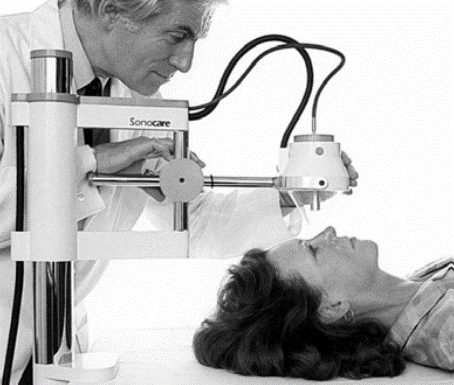
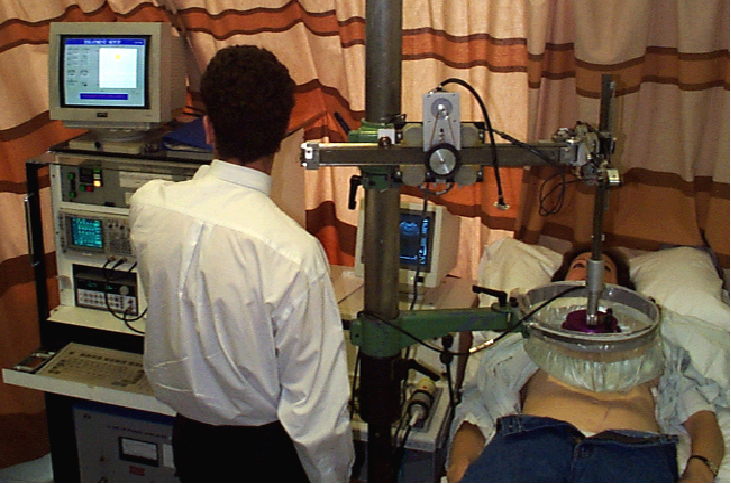
Where are we with clinical studies? Our first patient treatments at the Royal Marsden used a homemade device (right) with a single-element focused bowl transducer, homemade water bath, and homemade system for moving the transducer. We used a cardiac ultrasound device that we swapped in and out for imaging. We used this system to treat 75 phase I/phase II patients with liver cancer.
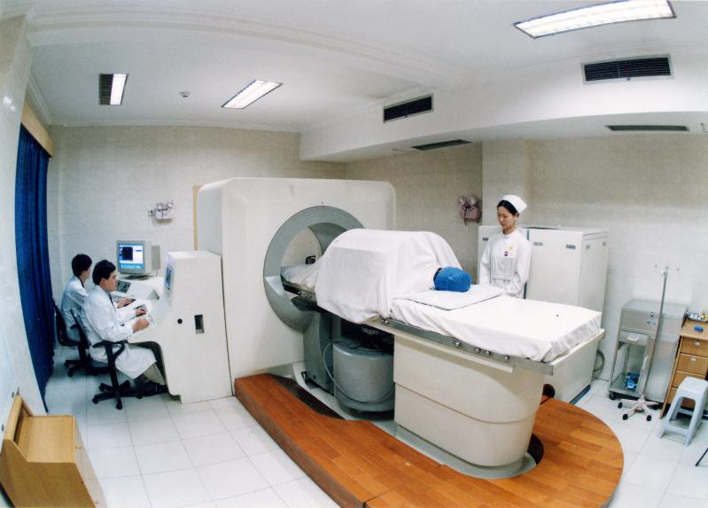 The Chongqing HAIFU device is an ultrasound-guided device. The image shows their 1999 system. It includes a system for moving the transducer, which sits under the bed where the patient is placed. The way in which the HIFU treatment is conducted, as I mentioned earlier, is by starting at the back of a tumor and working forward. The first treatment done in China using this device was to treat malignant bone tumors, such as osteosarcoma. 36 months after HIFU treatment – and this was done in 1998 – the patient showed complete response. These are very nice early results from the Chongqing group. The Chongqing HAIFU system has been used in China, at Oxford, and across Europe. It has been used to treat metastatic colorectal carcinoma, primary renal cell carcinoma, and pancreatic cancer. The absence of contrast agent uptake shows the treatment effect.
The Chongqing HAIFU device is an ultrasound-guided device. The image shows their 1999 system. It includes a system for moving the transducer, which sits under the bed where the patient is placed. The way in which the HIFU treatment is conducted, as I mentioned earlier, is by starting at the back of a tumor and working forward. The first treatment done in China using this device was to treat malignant bone tumors, such as osteosarcoma. 36 months after HIFU treatment – and this was done in 1998 – the patient showed complete response. These are very nice early results from the Chongqing group. The Chongqing HAIFU system has been used in China, at Oxford, and across Europe. It has been used to treat metastatic colorectal carcinoma, primary renal cell carcinoma, and pancreatic cancer. The absence of contrast agent uptake shows the treatment effect.
MR-guided HIFU is nicely illustrated using the work of Alessandro Napoli in Rome, who has treated bone metastases and osteoid osteomas. MR guidance is important for visualizing bone and breast tumors. It is important to have MR to see the structure of the bone, such as the nidus of the osteoma that has been destroyed.
Both MR- and ultrasound-guided HIFU are being used to treat uterine fibroids. An early paper from Claire Tempany and Kullervo Hynynen in 2003 shows the positioning of the focal spots and the absence of contrast, following treatment.
Nostradamus said that sound waves would kill cancer between 1992 and 1998. We did our first treatment at ICR in February 1997, so his prediction of the time that it was going to happen was pretty good. We did not actually cure cancer, but we were looking at proof of principle and doing partial treatments.
The First Clinical Treatment Systems
The first purposefully built HIFU unit that we know of in the world was one at Oxford that was located in an old Nissen hut and opened by Sir Peter Morris in 2002. Pictured below with that system are Professors David Cranston, Zhibiao Wang from Chongqing HAIFU, Peter Morris (professor of surgery at Oxford), and Katrina Leeder from “Airline,” a popular soap opera in those days. The ultrasound transducer sat in a water bath underneath the patient, who was lying on the bed. These pioneers of the Oxford unit included James Kennedy, who set the whole thing up, earned his PhD, and performed the first treatment.

 The history of HIFU also includes David Wild, a businessman who heard about HIFU, heard about the Chongqing group, and worked diligently to bring the Chinese system to the UK. Because he knew David Cranston, it went to Oxford. And the rest is history. Feng Wu is one of the clinical pioneers of HIFU, and he worked with the team of this unit to conduct liver treatments and enable the Chongqing HAIFU system to obtain the CE mark for treating the liver. That made it much easier for others to obtain CE marks and FDA approval. Chongqing HAIFU’s current system, the JC200, looks a bit more sophisticated than the earlier device.
The history of HIFU also includes David Wild, a businessman who heard about HIFU, heard about the Chongqing group, and worked diligently to bring the Chinese system to the UK. Because he knew David Cranston, it went to Oxford. And the rest is history. Feng Wu is one of the clinical pioneers of HIFU, and he worked with the team of this unit to conduct liver treatments and enable the Chongqing HAIFU system to obtain the CE mark for treating the liver. That made it much easier for others to obtain CE marks and FDA approval. Chongqing HAIFU’s current system, the JC200, looks a bit more sophisticated than the earlier device.
The first company to produce an MR-guided system was Insightec with the Exablate. Kobi Vortman worked with Ferenc Jolesz at Brigham and Women’s Hospital in Boston (pictured at right) to develop the system that slots into the bore of the magnet. The system initially worked mainly with GE magnets but is now compatible with other magnets.
Another MR-guided focused ultrasound system, the Sonalleve, was originally developed by Philips but has been sold to Profound Medical. This is the one that we have at the Royal Marsden Hospital in Sutton. Thomas Andreae helped us get it going with people from the Focused Ultrasound Foundation. Nandita deSouza is the clinician who helped us do all of our clinical trials.
 The most HIFU clinical experience to date is in treating the prostate, and most treatments are performed under ultrasound guidance, although MR guidance is also now available. The two companies that launched prostate HIFU are EDAP Technomed, which produced the Ablatherm device – and now also the Focal One device – and Focused Surgery, which became SonaCare Medical and produced the Sonablate device. Emmanuel Blanc and Jean-Yves Chapelon from EDAP have provided images to show how the Ablatherm device developed from 1993 to 1995. The therapy and imaging transducers were originally separate. The number of prostate treatments has substantially risen over the years, up to 30,000 treatments by 2012. Comparing the early Ablatherm device to the current Focal One device shows a more sophisticated version of the same thing. What has changed the most is the imaging and the display – in the user interface. Sonacare’s Sonablate system – and I’m grateful to Naren Sanghvi for these slides – was, again, a similar, smaller device that was first developed for treating benign prostate hyperplasia in 1992 and for treating prostate carcinoma in 1995.
The most HIFU clinical experience to date is in treating the prostate, and most treatments are performed under ultrasound guidance, although MR guidance is also now available. The two companies that launched prostate HIFU are EDAP Technomed, which produced the Ablatherm device – and now also the Focal One device – and Focused Surgery, which became SonaCare Medical and produced the Sonablate device. Emmanuel Blanc and Jean-Yves Chapelon from EDAP have provided images to show how the Ablatherm device developed from 1993 to 1995. The therapy and imaging transducers were originally separate. The number of prostate treatments has substantially risen over the years, up to 30,000 treatments by 2012. Comparing the early Ablatherm device to the current Focal One device shows a more sophisticated version of the same thing. What has changed the most is the imaging and the display – in the user interface. Sonacare’s Sonablate system – and I’m grateful to Naren Sanghvi for these slides – was, again, a similar, smaller device that was first developed for treating benign prostate hyperplasia in 1992 and for treating prostate carcinoma in 1995.
Professor Michael Marberger in Vienna was one of the clinical pioneers for treating prostate cancer. Professor Marberger and Dr. Donahue conducted their clinical studies with the Sonablate system. The current system looks much more sophisticated and professional, but it’s not that different, really. It is the imaging processing that’s changed.
There are now two MR-guided systems for prostate. The TULSA system from Profound Medical uses a transurethral rather than transrectal approach. Insightec’s Exablate prostate system is an MR-guided transrectal device.
HIFU Today
Where are we now? The current status of HIFU is incredibly different from that in the 1950s. The marjority of treatments – 40% of them – have been for uterine fibroids, which is something I decided not to discuss today. There is now a wide range of applications for HIFU.
Conclusion
 I’ll conclude by thanking pioneers from the past, present, and future. I have shown some of the photographs I found in my archives of people — from Bill Fry and Guy Vallancien to the present day. I particularly like this photo of the first three presidents of ISTU (Larry Crum, Kullervo Hynynen, and myself), which I have captioned “See No Evil, Say No Evil, Hear No Evil.”
I’ll conclude by thanking pioneers from the past, present, and future. I have shown some of the photographs I found in my archives of people — from Bill Fry and Guy Vallancien to the present day. I particularly like this photo of the first three presidents of ISTU (Larry Crum, Kullervo Hynynen, and myself), which I have captioned “See No Evil, Say No Evil, Hear No Evil.”
Thank you very much.
*All images reproduced in this article are were sourced from Professor Gail ter Haar’s webinar on December 10, 2020, entitled “History of Focused Ultrasound” and presented as part of the International Society for Therapeutic Ultrasound’s “ISTU On-Air” Series. Many images are sourced individually in the presentation, which is available here.