![]() Profound Medical recently announced that its TULSA-PRO® device has earned 510(k) clearance by the US Food and Drug Administration (FDA) to ablate prostate tissue.
Profound Medical recently announced that its TULSA-PRO® device has earned 510(k) clearance by the US Food and Drug Administration (FDA) to ablate prostate tissue.
TULSA-PRO is the first transurethral ultrasound device to earn FDA approval for the ablation of prostate tissue. It uses real-time MRI guidance and temperature feedback control combined with radiation- and incision-free ultrasound to allow physicians to predictably ablate whole-gland or partial prostate tissue.
The decision was based on positive results from the company’s international TACT pivotal clinical trial of 115 patients with organ-confined prostate cancer. In that study, patients had a median prostate-specific antigen (PSA) reduction of 95% to nadir of 0.34 ng/ml. Furthermore, patients experienced a low incidence of side effects associated with traditional prostate surgery, such as urinary incontinence and erectile dysfunction.
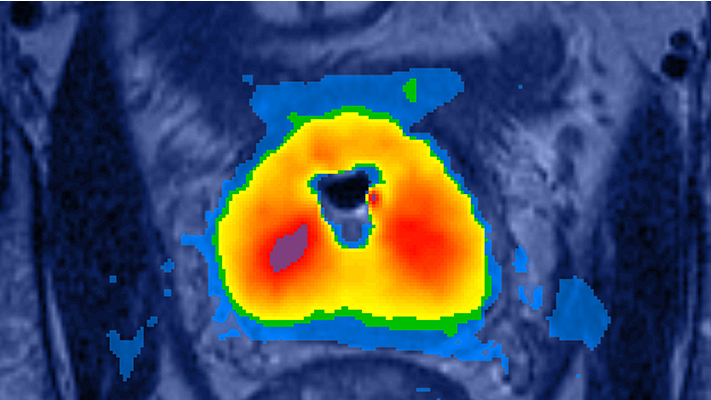 The transurethral approach provides better access to the entire prostate gland. This is important for patients who have prostate enlargement, because with existing therapies, they may need an additional procedure to reduce the size of the prostate. According to the company, in additon to treating almost any size prostate and having the ability to easily reach posterior and anterior portions of the gland, the transurethral approach helps to preserve the patient’s natural functions by actively cooling the urethra while delivering heat directly into the prostate and eliminating the need to deliver heat through the rectum. Since the ultrasound is directional and not focused, there is no possibility to leave cold spots between discrete sonications.
The transurethral approach provides better access to the entire prostate gland. This is important for patients who have prostate enlargement, because with existing therapies, they may need an additional procedure to reduce the size of the prostate. According to the company, in additon to treating almost any size prostate and having the ability to easily reach posterior and anterior portions of the gland, the transurethral approach helps to preserve the patient’s natural functions by actively cooling the urethra while delivering heat directly into the prostate and eliminating the need to deliver heat through the rectum. Since the ultrasound is directional and not focused, there is no possibility to leave cold spots between discrete sonications.
Because the FDA’s ruling allows the ablation of both malignant and benign prostate tissue, physicians could use it to treat a variety of prostate diseases like prostate cancer and benign prostatic hyperplasia (BPH). The TULSA-PRO takes advantage of in-bore MRI-guidance, providing physicians with high quality images for customized treatment planning and more importantly, real-time MR thermometry feedback control for predictable ablation of the targeted prostate tissue.
Profound Medical is the latest ultrasound device company to earn approval to ablate prostate tissue in the US. In 2015, SonaCare Medical and EDAP-TMS announced approval of their devices, both of which utilize a transrectal approach and ultrasound imaging guidance instead of MRI.
Profound’s TULSA-PRO also earned CE approval in Europe in April 2016.
“The feedback from physicians using the system in Europe, and from key opinion leaders in the United States who have first-hand experience with the technology as TACT trial investigators, has been very positive, particularly regarding the ease-of-use, efficiency and flexibility based on patient needs; in addition to its excellent patient tolerability and short recovery periods,” explains Profound’s CEO, Arun Menawat. “Given this interest and feedback, we are very much looking forward to working with our strategic partners, Philips and Siemens Healthcare, in concert with our direct sales and marketing teams, to prepare for the US commercial launch of TULSA-PRO® in Q4-2019.”
