 The first of six patients has been enrolled in a new trial at Linkou Chang Gung Memorial Hospital in Taiwan to evaluate the feasibility, safety, and preliminary efficacy of focused ultrasound to open the blood-brain barrier (BBB) and facilitate the delivery of the chemotherapy drug bevacizumab in patients with recurrent glioblastoma.
The first of six patients has been enrolled in a new trial at Linkou Chang Gung Memorial Hospital in Taiwan to evaluate the feasibility, safety, and preliminary efficacy of focused ultrasound to open the blood-brain barrier (BBB) and facilitate the delivery of the chemotherapy drug bevacizumab in patients with recurrent glioblastoma.
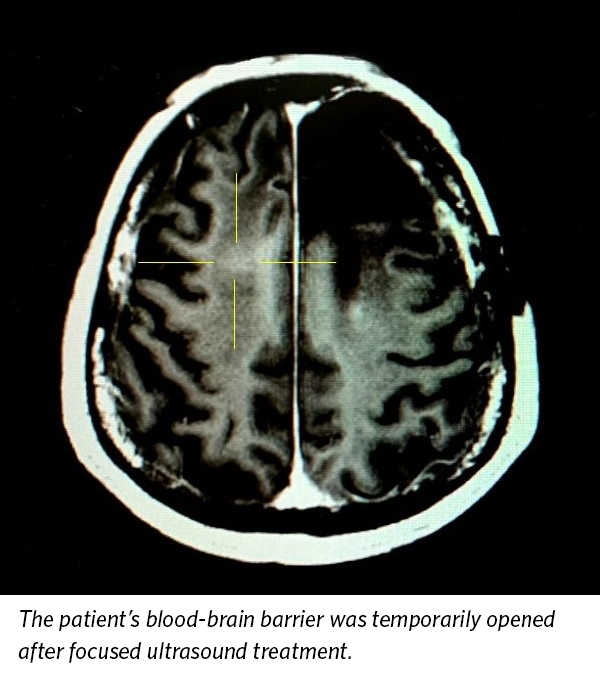
The BBB is a protective layer of tightly joined cells that lines the blood vessels in the brain and prevents harmful substances, such as toxins and infectious agents, from diffusing into the surrounding brain tissue. However, it can also prevent therapeutic agents – like chemotherapy – from getting into the brain.
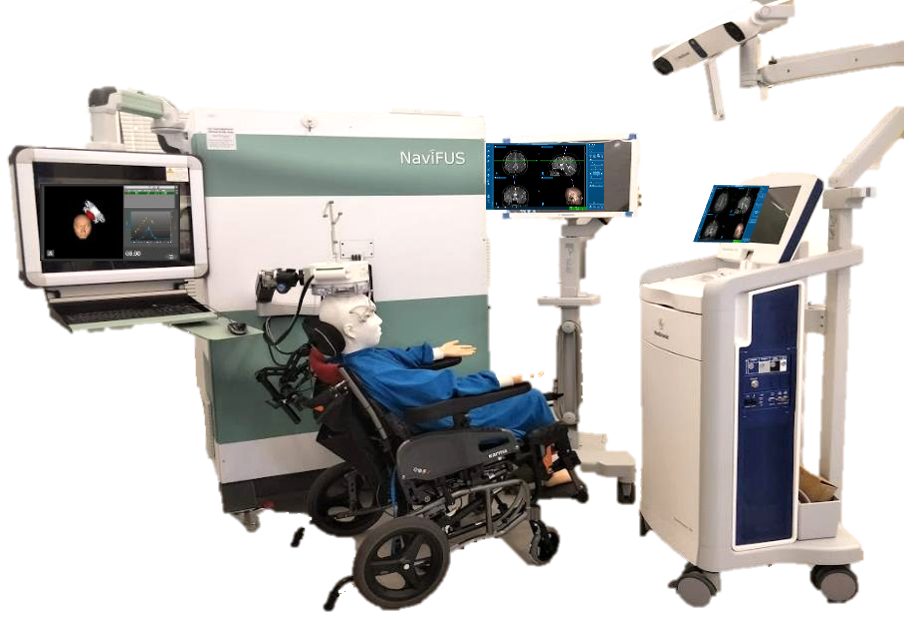
Led by neurosurgeon Kuo-Chen Wei, MD, the trial is employing the proprietary NaviFUS System (pictured at right) to noninvasively and repeatedly open the BBB to enable bevacizumab to enter the brain in higher concentrations. Patients will undergo biweekly bevacizumab and focused ultrasound treatment for up to 34 weeks or until further disease progression.
Unlike other focused ultrasound devices that use real-time MR technology to guide brain treatments, the NaviFUS System uses neuronavigation and prior patient CT/MR images to direct the focused ultrasound energy. The device also includes a real-time acoustic emission/reflection monitoring function that can personalize a safe and optimal level of focused ultrasound energy treatment for each patient.
This trial builds upon the results from an earlier study that demonstrated the device could be used to open the BBB safely in patients who did not receive the drug.
 “The goal of the current study is to investigate the possibility of delivering a large molecular weight drug to the brain in higher concentrations using focused ultrasound technology.” said NaviFUS Chief Executive Officer Arthur Lung, PhD. “Should we observe positive results from the study, we expect it will be a good model for further extension to other biologicals, antibodies, antibody-drug conjugates, and cell therapies. This is an important step in meeting the goal of our company, which uses an innovative and safe way to help treat Central Nervous System (CNS) diseases in the future.”
“The goal of the current study is to investigate the possibility of delivering a large molecular weight drug to the brain in higher concentrations using focused ultrasound technology.” said NaviFUS Chief Executive Officer Arthur Lung, PhD. “Should we observe positive results from the study, we expect it will be a good model for further extension to other biologicals, antibodies, antibody-drug conjugates, and cell therapies. This is an important step in meeting the goal of our company, which uses an innovative and safe way to help treat Central Nervous System (CNS) diseases in the future.”
“This trial uses an innovative device to open the blood-brain barrier repetitively,” says Foundation Chairman Neal F. Kassell, MD. “It is an important step in the path to developing novel approaches for delivering a variety of therapeutic agents to the brain. This can facilitate the treatment of primary and metastatic brain tumors as well as neurodegenerative diseases for which there are few other treatments, including Parkinson’s, Alzheimer’s, and ALS.”
The current study is being supported by the Focused Ultrasound Foundation.
