Key Points
- The US Food and Drug Administration (FDA) established the Breakthrough Devices Program in 2018 to allow fast-tracking of certain novel devices.
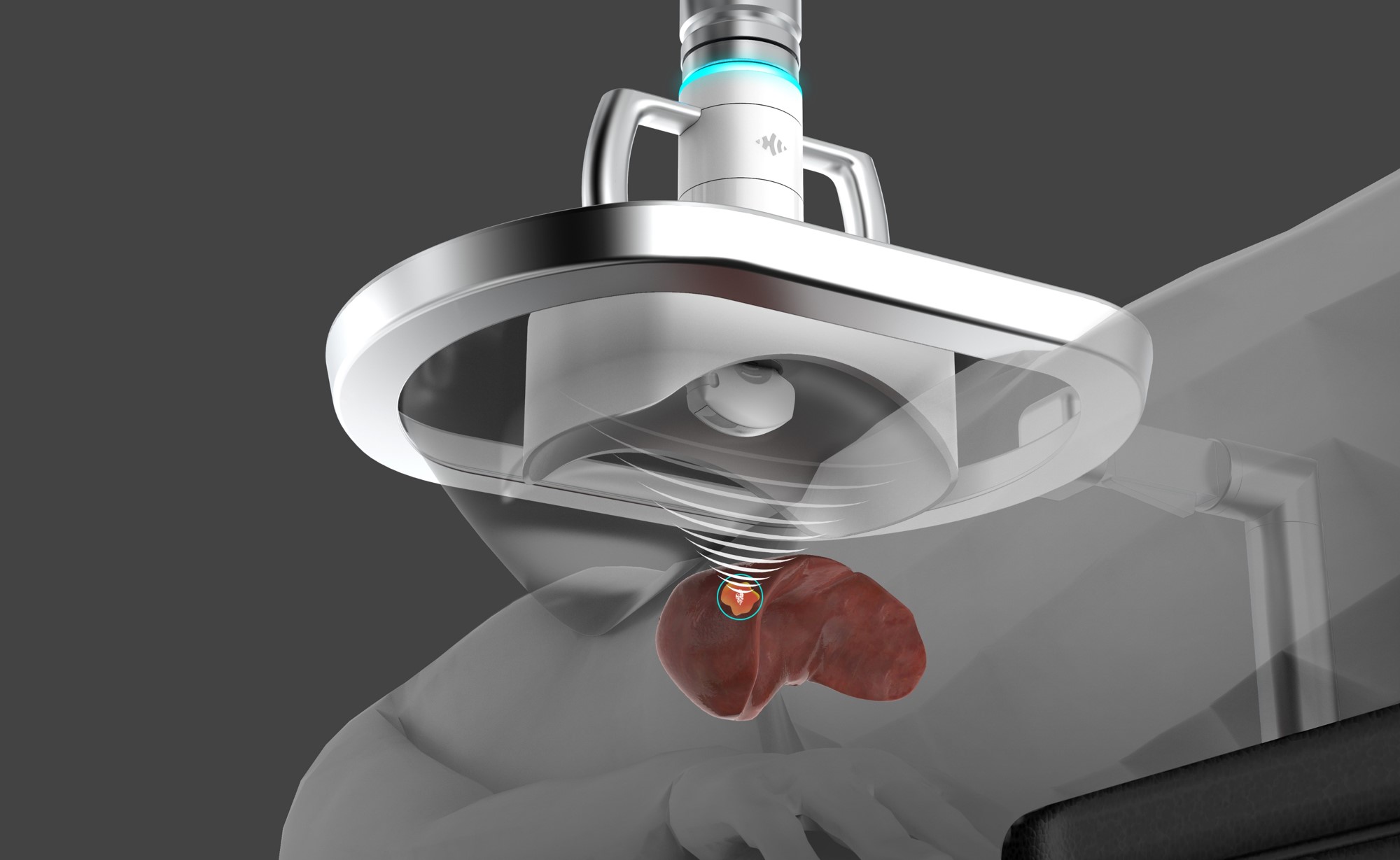
- The HistoSonics platform, called EdisonTM, uses histotripsy to noninvasively and mechanically destroy tissue.
- The procedure is being investigated in patients with liver tumors in clinical trials in Europe and the US.
 HistoSonics recently announced that their novel therapy platform has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA).
HistoSonics recently announced that their novel therapy platform has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA).
The Breakthrough Devices Program was established by the FDA in 2018 to allow fast-tracking of certain novel devices.
“The program offers device manufacturers an opportunity to work directly with the FDA through the premarket review stage of the approval process,” explains Jessica Foley, PhD, the Foundation’s Chief Scientific Officer and Managing Director of Government Affairs. “The goal is to expedite patient access to devices that provide more effective treatment or diagnosis, through a more rapid assessment and review process, while preserving the FDA’s standards of approval.”
The HistoSonics platform, called EdisonTM, uses histotripsy to noninvasively and mechanically destroy tissue in the liver. The procedure is currently being investigated in patients with primary and metastatic liver tumors in two similar clinical trials in Europe and the US – both are called #HOPE4LIVER. Eight sites in the US and five in Europe are currently enrolling patients.
“The Breakthrough Device Designation is a significant milestone for our company and validates our belief that our platform offers significant advantages over existing approved or cleared alternatives, per FDA requirements,” said Mike Blue, President and CEO of HistoSonics. “Early and ongoing clinical results are promising and suggest that our ability to precisely destroy targeted liver tissue, completely noninvasively, and without the challenges associated with ionizing radiation or other locoregional therapies, provides advantages to patients and physicians that don’t exist today, and we look forward to working with the FDA to make the technology accessible as quickly as possible.”
Earlier this year, the company also announced that the American Medical Association had issued a new Current Procedural Terminology (CPT®) procedure code for histotripsy of the liver – a first for a histotripsy-based focused ultrasound therapy.
Read the Press Release from HistoSonics >
More about the Breakthrough Device Program
The Breakthrough Device Program replaces the Expedited Access Pathway (EAP) and Priority Review for medical devices programs, as the EAP did not include 510(k) applications and the Priority Review is now limited only to drugs, and not devices.
Devices that are subject to PMA, 510(k), or requests for de novo designation are eligible for Breakthrough Device designation if they meet two criteria:
- The device provides a more effective treatment or diagnosis for a life threatening or debilitating human disease or condition than previous therapies.
- The device meets at least one of the following: a) represents breakthrough technology, b) no approved or cleared alternatives currently exist, c) provides a significant advantage over current cleared or approved alternatives, d) the device’s availability is in the best interest of patients.
